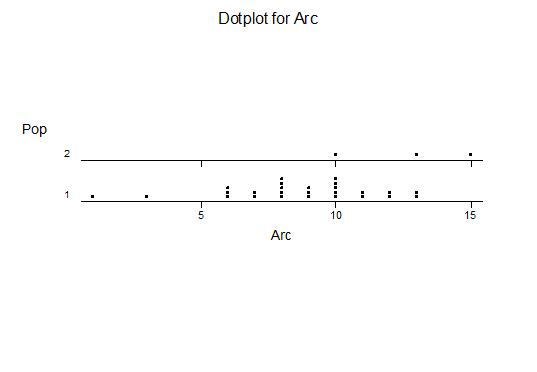
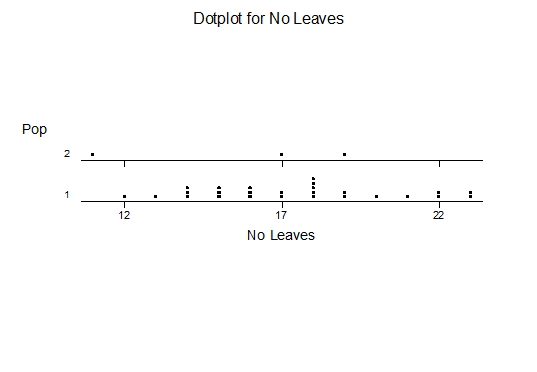
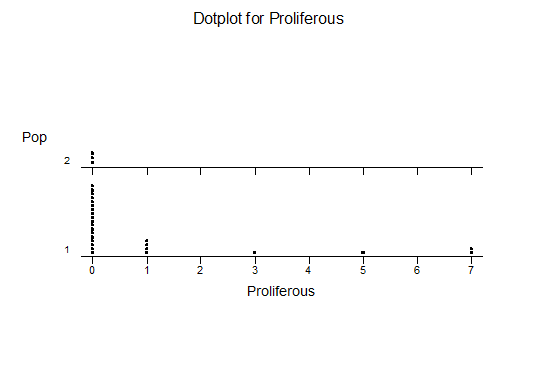
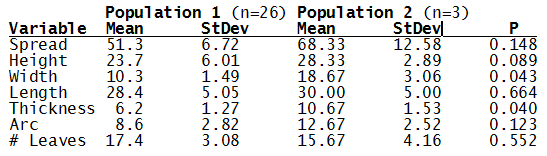
Bayer Accessions
A plant classification is essentially based on herbarium record, which is the prime means of verification. However, it is not practicable for such a record to embrace all records nor record all the variation that has been observed. In recent time, I have not deposited new material and the only record of accessions other than living specimens, is a photographic one.
This record of my collections is in four parts:-
- Un-numbered herbarium specimens.
- Collections under Karoo Botanic garden numbers (KG).
- Collections in a personal register (MBB).
- A record of J.D.Venter numbers for MBB collections
1. Un-numbered collections.
The following collections of mine exist as herbarium specimens in the Compton herbarium, and which have no Karoo Botanic Garden nor a personal accession number:-
– decipiens var. cyanea 3222 DC Trakaskuilen.
The original collection of two plants was made in about 1988, which I later accessioned as 6885; and I collected seed again in 2000 under the number 7025.
– mucronata var inconfluens 3321 AC 8km W Ladismith.
This is a collection I often refer to when I discuss ecotypes. This collection refers to one on shales at Dwarsrivier, whereas a very short distance away in the wetter sandstone gorge, there is the var. habdomadis.
– mirabilis var. consanguinea 3419 BA Die Galg.
Also an old collection to which George Payne directed me in 1971. I named it to include a collection by J.D.Venter lower down the Gobos River (Boesmanpad), a collection by Dawn Schwegmann along that gorge, a collection by P.V.Bruyns from the upper Dwarsriverkloof, and one by the two of us lower down that stream.
– limifolia var. limifolia 2631 BD Mbuluzi gorge, Blue Jay Ranch, Stegi.
Collected in about 1962 in the company of my friend M.G.Murdoch. The plants were very abundant and may have been one very stoloniferous clone! Plants were widely distributed and are still in cultivation.
– mucronata var. inconfluens 3321 CA 9km SW Ladismith.
The only collection I can recall is one I made near the road junction to Garcia Pass, and the plants were small marumiana-like. I would have difficulty classifying that collection again and would probably consider it to be better placed under arachnoidea var. nigricans!
– marginata 3320 CC NE Ashton.
From the site where pumila and marginata co-occur.
– heidelbergensis var. scabra 3420 AD W Kathoek.
When I first saw this population I thought it to be a form of maraisii as I then did not have material to suggest that heidelbergensis was such a strong element.
– cooperi var. pilifera 3227 BD Fort Murray Bridge.
A collection probably still in cultivation and I saw very similar plants both west and north of Kingwilliamstown.
– reticulata var. reticulata 3319 DB Buitenstekloof.
George Payne explained to me that this was the site of H. intermedia of Von Poellnitz, and a full explanation is given in my essay on that name.
– parksiana 3422 AA Botteliersberg.
There is a back road from Little Brak to Great Brak and there used to be a sheet of rock projecting into the road. It was on this sheet of rock where parksiana grew like a weed. Unfortunately the rock slab fell prey to road straightening and few plants survive there now.
– pygmaea var. argenteomaculata 3421 BB Cooper Siding.
From the site of Smith’s original accession and there are still many plants there.
– mutica var. mutica 3419 DB Klipdale.
An accession never brought into cultivation.
– pumila 3319 CD Lemoenpoort.
An unusual population from which plants are in cultivation. The plants tend to have a very purple colour and the tubercles are sparser than normal.
– sordida var. sordida 3325 AC Brakfontein.
The significance of this collection is the co-occurrence with the then little known Aloe bowiea. The collection is not in cultivation.
– pulchella var. pulchella 3320 CA Nougaspoort.
A species which is not very variable and there is nothing exceptional about this population.
– reticulata var. reticulata 3319 DC Dublin.
Very abundant between its westernmost occurrence at Ribbokop, to the west of this population, and Robertson to the east.
– herbacea var. paynei 3319 DD 1km S McGregor.
I do not recall making such a collection although I have seen plants to the south-east and also north of the town.
– pygmaea var. argenteomaculata 3421 BB Humor.
My early collecting was really aimed at getting an overview of the genus and familiarising myself with Smith’s material. So when I first saw plants at Humor I was satisfied that they were similar to the Cooper Siding plants and I did not make a record. Now of course every population has a significance and I could not re-locate my original sighting east of this one. The Humor plants generally are more marked than the Cooper Siding ones.
– marginata 3420 AC Adoonskop.
I also saw the species here early in my collecting and made no record. The plants were grazed to ground level and were much smaller than elsewhere. I have since collected it and grown it from seed as 6633, and D.M.Cumming has collected a smaller version from nearby there. I think I noted an Otzen record as east of Napier, when in fact the record was for west of the town. It has not, to my knowledge, been found there again.
– pulchella var. pulchella 3320 CB SW Anysberg.
The significance of this collection is that the plants are small, very proliferous, and very green.
– pumila 3319 BC Osplaas, DeDoorns.
The distribution is odd because it is outside the Worcester-Robertson Karoo, but there are of course collections from as far afield as Matjesfontein near Laingburg, and the Anysberg Nature reserve.
– kingiana 3422 AA Little Brak, Barswel.
A strange population which may still be exemplified in cultivation at the Karoo Botanic garden. Some of the plants were without tubercles at all.
– semiviva 3120 CD SE Middelpos.
The plants were unusually robust for the species and this is also the furthest west the species is known to me.
– mutica var. mutica 3420 AB Crodini.
I do not recall this locality. On one trip I made to Bredasdorp I saw many different populations of mutica which I did not record. This may be one of them, or it may be specifically one from south of Napky which I have grown from seed as 7029.
1404a cooperi var. tenera 3325 CB Groendal Dam.
I was actually exploring asclepiads when I saw this plant. The remote and rugged terrain there needs better exploration.
2. Karoo Garden (KG) Collections.
The following are only the collections which were reduced to herbarium record and which do not, in the main, replicate other earlier records. Some of these collections were made in the company of others company and I regret that this is not better conveyed:-
623/69 mutica var. mutica 3420 AC Soesriver.
627/69 retusa 3421 AB Near, Riversdale.
628/69 mirabilis var. badia 3419 BD Napier.
630/69 maraisii var. maraisii 3319 DD W Robertson.
631/69 turgida var. suberecta 3422 AA Brandwacht.
640/69 arachnoidea var. arachnoidea 3319 CB NE. Worcester.
662/69 reticulata var. reticulata 3319 DA Keeromskloof.
669/69 maculata var. maculata 3319 CB Brandvlei Dam.
681/69 mirabilis var. triebneriana 3419 BD Mierkraal, Napier.
682/69 mirabilis var. triebneriana 3419 AB Uitkyk.
688/69 maraisii var. maraisii 3319 DD SW Robertson.
692/69 mirabilis var. triebneriana 3419 BA Near Genadendal.
695/69 herbacea var. paynei 3319 DD McGregor.
26/70 mirabilis var. triebneriana 342O AA Stormsvlei.
28/70 mirabilis var. triebneriana 3419 AB N Uitkyk.
30/70 mirabilis var. triebneriana 3419 BA near Greyton.
31/70 mirabilis var. triebneriana 3419 BA near Greyton.
32/70 mirabilis var. beukmannii 3419 BA Skuitsberg.
34/70 turgida var. longibracteata 3420 AC N Bredasdorp.
35/70 maraisii var. maraisii 3420 AC N Bredasdorp.
36/70 heidelbergensis var. minor 3420 CA Rooivlei.
46/70 maraisii var. maraisii 3319 DD Roberts.to Bonnievale.
118/70 arachnoidea var. arachnoidea 3319 DA Effata.
163/70 maraisii var. maraisii 3319 DD S Goudmyn.
166/70 herbacea var. herbacea 3319 CB Brandwacht.
175/70 reticulata var. reticulata 3319 DC Rooiberg.
200/70 retusa 3421 AA W Riversdale.
202/70 magnifica var. atrofusca 3421 AA Spitzkop, W Riversdal.
218/70 herbacea var. herbacea 3319 DC Mowers.
224/70 maraisii var. meiringii 3320 CC E Bonnievale.
231/70 decipiens var. decipiens 3323 AD Skerpkop, E Willowmore.
310/70 bolusii var. blackbeardiana 3227 AC SW Cathcart.
311/70 bolusii var. blackbeardiana 3227 AA Imvani.
312/70 bolusii var. blackbeardiana 3126 DD Bowes’ farm.
315/70 decipiens var. virella 3324 AA Campherpoort.
323/70 mucronata var. morrisiae 3321 BC Bergplaas.
329/70 herbacea var. herbacea 3319 DD Koningsrivier.
336/70 cooperi var. gracilis 3326 AB Hellspoort.
382/70 cooperi var. pilifera 3326 DC Brigadoon.
383/70 cooperi var. pilifera 3326 DA Peninsula.
392/70 cooperi var. cooperi 3227 AC W Cathcart.
393/70 bolusii var. blackbeardiana 3227 AC SW Cathcart.
402/70 springbokvlakensis 3324 BD Springbokvlakte.
806/70 longiana ‑ ‑ SE Hankey.
2/71 maraisii var. meiringii 3320 CC W Bonnievale.
7/71 maraisii var. meiringii 3320 CC W Bonnievale.
9/71 maraisii var. meiringii 3320 CC W Bonnievale.
36/71 arachnoidea var. arachnoidea 3319 BD Tonnel Stn.
77/71 mucronata var. rycroftiana 3321 DD 23km E Ladismith.
83/71 magnifica var. magnifica 3421 AA S Riversdale.
86/71 turgida var. longibracteata 3421 AD Stilbay.
91/91 retusa 3421 AB E Riversdale.
92/71 magnifica var. magnifica 3420 BA S Tradouw Pass.
93/71 variegata var. variegata 3421 AB Hoekraal.
94/71 turgida var. longibracteata 3421 AC Brakfontein.
98/71 chloracantha var. subglauca 3422 AA E Great Brak.
115/71 arachnoidea var. nigricans 3321 DB Warmwaterbron.
118/71 emelyae var. major 3321 CC Muiskraal.
120/71 mucronata var. morrisiae 3322 CB Hazenjacht.
156/71 retusa 3421 AB Blinkbonnie, E Riversdale.
166/71 arachnoidea var. nigricans 3321 CC Springfontein.
167/71 arachnoidea var. nigricans 3321 CC W Springfontein.
209/71 mutica var. nitida 3420 BB Kransriviermond.
275/71 retusa
311/71 magnifica var. acuminata 3421 BD N Gouritzmond.
324/71 reticulata var. subregularis 3319 DA Boskloof.
326/71 maraisii var. maraisii 3420 AA Rondeheuwel.
327/71 mutica var. mutica 3420 AC N Kykoedie.
388/71 arachnoidea var. setata 3321 DA E Vanwyksdorp.
345/71 maraisii var. maraisii 3319 DD W Robertson.
535/71 arachnoidea var. setata 3321 CB W Vanwyksdorp.
567/71 mucronata var. inconfluens 3321 AC Dwarsrivier.
568/71 mucronata var. morrisiae 3321 CB W Vanwyksdorp.
573/71 arachnoidea var. nigricans 3321 CB Ladismith to Vanwyksd.
593/71 arachnoidea var. setata 3321 CB E Ladismith.
6/72 cooperi var. leightonii 3327 BA Kaysers Beach.
47/72 cooperi var. tenera 3326 BA Plutosvale.
47/72a cooperi var. tenera 3326 BA Plutosvale.
85/72 mutica var. mutica 3420 AC Badjieskraal.
111/72 bayeri 3322 CA S Oudtshoorn.
114/72 emelyae var. comptoniana 3323 AD Georgida.
119/72 scabra var. morrisiae 3322 AD Schoemanspoort.
126/72 arachnoidea var. nigricans 3320 DD E Lemoenshoek.
138/72 emelyae var. major 3321 CD Sandkraal.
140/72 decipiens var. decipiens 3323 BB Constantia.
146/72 bayeri 3322 CB Doringkloof.
151/72 mucronata var. morrisiae 3322 CB Kamanassie Dam.
160/72 arachnoidea var. setata 3321 CB S Ladismith.
160/72 arachnoidea var. setata 3322 BC Meiringspoort.
240/72 turgida var. turgida 3420 BB N Heidelberg.
241/72 turgida var. turgida 3420 BB N Heidelberg.
329/72 nortieri var. nortieri 3118 DC Die Kom.
125/73 arachnoidea var. nigricans 3321 DA S Calitzdorp.
155/73 fasciata 3325 CB Hillwacht.
193/73 cooperi var. gordoniana 3324 DD N Hankey.
311/73 magnifica var. acuminata 3421 AD N Gouritzmond.
100/74 blackburniae var. blackburniae 3321 AC W Ladismith.
107/74 magnifica var. magnifica 3420 BB SW Heidelberg.
109/74 mucronata var. morrisiae 3322 CA Volmoed.
100/740 blackburniae var. blackburniae 3321 DB Warmbaths.
411/75 chloracantha var. chloracantha 3321 DD N Herbertsdale.
436/75 decipiens var. cyanea 3323 AD Georgida.
441/75 arachnoidea var. nigricans 3321 BC N Calitzdorp.
89/76 reticulata var. hurlingii 3320 CC Goudmyn.
90/76 reticulata var. reticulata 3319 DC Rooiberg.
83/77 mutica var. mutica 3420 AC Kykoedie.
257/77 emelyae var. emelyae 3321 DC SE Vanwyksdorp.
3. M.B.Bayer personal numbers.
112 limifolia var. gigantea 2731 DA Nongoma.
121 maraisii var. notabilis 3319 DD Vinkrivier.
153 arachnoidea var. arachnoidea 3319 DB Buitenstekloof.
154 minima var. poellnitziana 3320 CC W Drew.
155 lockwoodii 3320 BB Floriskraal.
157 parksiana 3422 AA Dumbie Dykes.
158 floribunda var. floribunda 3420 BB N Heidelberg.
159 mucronata var. inconfluens 3321 BC Die Berg.
160 reticulata var. reticulata 3319 DC Ribbokkop.
161 herbacea var. herbacea 3319 DC Ribbokkop.
163 pulchella var. pulchella 3320 AC Avondrust, Touws River.
163 pubescens var. pubescens 3319 CB Sandberg.
164 maculata var. maculata 3319 CB Brandvlei Dam.
166 rossouwii var. rossouwii 3420 BA Oudekraalkop.
167 venosa ssp. woolleyi 3324 BD Kleinpoort
168 venosa ssp. venosa 3420 AB Swellendam.
169 attenuata ‑ ‑ ‑
170 glabrata ‑ ‑ ‑
171 arachnoidea var. nigricans 3322 AC Schoemanspoort.
171 mucronata var. mucronata 3322 AD Schoemanspoort.
172 marumiana var. marumiana 3226 AB Spring Valley.
173 bolusii var. bolusii 3225 CA NE Pearston.
174 marginata 3421 AB S Heidelberg.
175 monticola var. monticola 3322 DD Molen River.
886 fasciata 3325 CB Spring Range.
887 fasciata 3325 CC E Hankey.
1119 maculata var. maculata 3319 CB Audensberg.
1120 maculata var. maculata 3319 DA S Sandhills.
1128 pubescens var. livida 3319 CD S Lemoenpoort.
1145 maculata var. maculata 3319 CD Moddergat.
1194 coarctata var. coarctata 3326 DB Fairfax.
1208 maraisii var. notabilis 3319 DD Wolfkloof.
1209 maraisii var. notabilis 3319 DD Wolfkloof.
1210 maraisii var. maraisii 3319 DC Trappieskraalkloof.
1211 maraisii var. maraisii 3319 DD W Robertson.
1213 maraisii var. maraisii 3420 AA N Stormsvlei.
1214 maraisii var. meiringii 3320 CC W Bonnievale.
1215 maraisii var. maraisii 3319 DC Langkloof.
1216 maraisii var. maraisii 3319 DD Goudmyn.
1217 maraisii var. meiringii 3320 CC W Bonnievale.
1218 maraisii var. meiringii 3320 CC W Bonnievale.
1219 maraisii var. maraisii 3320 CC N Drew.
1220 maraisii var. maraisii 3319 DD Klaasvoogds.
1221 maraisii var. maraisii 3420 AD Juliusfontein.
1221 maraisii var. maraisii 3319 DD Skurweberg.
1222 maraisii var. maraisii 3319 DD S McGregor.
1351 coarctata var. adelaidensis 3326 AB Hellspoort.
1352 coarctata var. coarctata 3326 AB Hellspoort.
1354 coarctata var. adelaidensis 3326 BC NW Grahamstown.
1355 coarctata var. adelaidensis 3326 AA Willowfountain.
1357 coarctata var. coarctata 3326 BC Brooklands.
1358 coarctata var. coarctata 3326 BC Manley Flats.
1359 coarctata var. coarctata 3326 DA Kowie.
1360 coarctata var. coarctata 3326 BC Vaalvlei.
1361 coarctata var. coarctata 3326 DA S Vaalvlei.
1363 coarctata var. coarctata 3326 CB Salem to Alexandria.
1364 coarctata var. coarctata 3326 DA Hopewell, W Port Alfred.
1366 coarctata var. coarctata 3326 DA Hopewell, W Port Alfred.
1367 coarctata var. coarctata 3326 BA Rooidrift.
1368 coarctata var. coarctata 3326 BA E Plutosvale.
1370 coarctata var. coarctata 3326 CA Woodbury.
1371 coarctata var. coarctata 3325 DB Orlando.
1372 coarctata var. coarctata 3326 AD Yellowwoods.
1381 reinwardtii var. brevicula 3326 BD Frazer’s Camp.
1391 reinwardtii var. reinwardtii 3327 AA W Peddie.
1438 reticulata var. reticulata 3319 DC Rooikleigat.
1539 reticulata var. reticulata 3319 DC S Gemsbokkop.
1543 reticulata var. reticulata 3319 DD Wolfkloof, Robertson.
1558 emelyae var. multifolia 3321 CC Springfontein.
1615 arachnoidea var. nigricans 3321 CA SW Ladismith.
1616 arachnoidea var. nigricans 3321 DB Oudtshoorn to Calitzdorp.
1617 arachnoidea var. nigricans 3321 CA Adamskraal.
1618 mucronata var. habdomadis 3321 AC Dwarsrivier.
1619 arachnoidea var. namaquensis 3219 DD Ceres Karoo.
1620 cymbiformis var. cymbiformis 3326 BA E Fort Brown
1621 cooperi var. leightonii 3327 BA SW Paynes Hill.
1621 mucronata var. morrisiae 3321 DB Warmbron.
1622 cooperi var. pilifera 3326 AA Willowfountain.
1623 cooperi var. pilifera 3227 DA St Johns Drift.
1624 arachnoidea var. nigricans 3321 CA W Ladismith.
1628 mucronata var. inconfluens 3321 CA S Ladismith.
1652 arachnoidea var. namaquensis 2817 CD Kliphoogte.
1661 venosa ssp. tessellata 2817 CD Kouefontein.
1674 arachnoidea var. namaquensis 2917 AB Karrachabpoort.
1698 heidelbergensis var. toonensis 3420 BB Matjestoon.
1700 heidelbergensis var. scabra 3420 AB Leeurivier.
1701 mucronata var. rycroftiana 3321 DC VanWyksdorp to Herbertsdale.
1702 zantneriana var. minor 3323 BB near Miller Stn.
1703 maraisii var. maraisii 3319 DD W Robertson.
1704 arachnoidea var. nigricans 3320 DD Warmwaterberg.
1706 cymbiformis var. setulifera 3228 BA S Mooiplaas.
1707 maraisii var. maraisii 3319 DD Muiskraalkop.
1708 maraisii var. maraisii 3320 CC N Ashton.
1963 arachnoidea var. scabrispina 3320 CA Nougaspoort.
1986 mucronata var. inconfluens 3320 DA Anysberg Pass.
1995 herbacea var. herbacea 3319 CD W Doornrivier.
1996 herbacea var. herbacea 3319 CD Lemoenpoort.
1997 herbacea var. herbacea 3319 DC Wansbek.
2011 marumiana var. marumiana 3124 CC Murraysburg.
2022 bolusii var. bolusii 3224 BA Graaff‑Reinet.
2024 bolussii var pringlei 3225 CB Bruintjieshoogte.
2038 marumiana var. marumiana 3126 BD N Tarkastad.
2052 angustifolia var. baylissii 3325 BC Oudekraal.
2070 decipiens var. virella 3224 DB Ebenezer.
2071 bolusii var. bolusii 3224 DD Lootskloof.
2072 bolusii var. bolusii 3224 BA Graaff‑Reinet.
2074 decipiens var. virella 3324 AA Campherpoort.
2076 decipiens var. minor 3324 AA Grootriver.
2083 decipiens var. cyanea 3323 CA N Uniondale.
2086 arachnoidea var. aranea 3322 DC Ganskraal.
2093 pulchella var. pulchella 3320 AB SE Konstabel Stn.
2099 mucronata var. morrisiae 3322 CA S Oudtshoorn.
2105 arachnoidea var. scabrispina 3320 BA N Baviaans Stn.
2117 viscosa 3220 DD N Laingsburg.
2124 arachnoidea var. scabrispina 3220 DC N Laingsburg.
2131 viscosa 3220 DD N Laingsburg.
2177 maraisii var. maraisii 3320 CC Goedverwacht.
2187 reticulata var. reticulata 3319 DD Wolfkloof, Robertson.
2241 pygmaea var. pygmaea 3422 AA Great Brak.
2271 maraisii var. maraisii 3319 DD Houtbaaikloof.
2287 pygmaea var. pygmaea 3422 AA W Great Brak.
2289 pygmaea var. pygmaea 3422 AA Dumbie Dykes.
2311 floribunda var. dentata 3421 BA Wydersrivier.
2347 marumiana var. marumiana 3224 AB Valley of Desolation.
2350 nigra var. nigra 3224 AB St Olives.
2356 marumiana var. marumiana 3124 DC Aasvoelkrans.
2372 venosa ssp. tessellata 3222 BC E Molteno Pass.
2373 marumiana var. marumiana 3222 BA Molteno Pass.
2377 decipiens var. cyanea 3221 CB W Merweville.
2380 bolusii var. bolusii 3124 CC W Graaff‑Reinet.
2385 venosa ssp. tessellata 3223 AA Nelspoort.
2389 bolusii var. bolusii 3123 DD E Murraysberg.
2406 semiviva 3222 BC S Beaufort West.
2418 arachnoidea var. nigricans 3321 CD W Vanwyksdorp.
2419 arachnoidea var. nigricans 3321 CD S Vanwyksdorp.
2420 turgida var. longibracteata 3420 AB SW Swellendam.
2421 herbacea var. herbacea 3319 CB W Worcester.
2422 herbacea var. herbacea 3319 CB SE Brandvlei Dam.
2423 magnifica var. acuminata 3421 BD N Gouritzmond.
2424 wittebergensis 3320 BC SW Laingsburg.
2425 lockwoodii 3320 BB Ezelsfontein.
2453 marumiana var. archeri 3221 CA Langberg.
2453 mirabilis var. beukmannii 3419 BA Skuitsberg.
2453 mirabilis var. triebneriana 3419 BA Dagbreek.
2469a nigra var. diversifolia 3221 CB W Merweville.
2526 mutica var. mutica 3420 AA Rietfontein.
2533 turgida var. longibracteata 3420 BC Diepkloof, S Malgas.
2547 heidelbergensis var. scabra 3420 AC Brakfontein.
2550 heidelbergensis var. heidelberg 3420 BB E Heidelberg.
2551 variegata var. modesta 3420 AD SW Kathoek.
2556 heidelbergensis var. scabra 3420 AD Beyersdal.
2564 variegata var. hemicrypta 3420 BC NE Potberg.
2574 arachnoidea var. namaquensis 3219 DC Skitterykloof.
2579 herbacea var. lupula 3319 CD Wolfkloof, Villiersdorp.
2582 pulchella var. pulchella 3319 BD W Touws River.
2591 maculata var. maculata 3319 CB NE Brandvlei Dam.
2665 magnifica var. atrofusca 3421 AA Droerivier.
2670 venosa ssp. venosa 3420 BC Malgas.
2672 turgida var. longibracteata 3421 AC Duiwenhoksriver.
2697 herbacea var. herbacea 3319 DC Keerweerder, Jonaskop.
2716 arachnoidea var. nigricans 3321 CA Winkelplaas.
3363 monticola var. monticola 3323 AD Georgida.
3375 cooperi var. viridis 3324 BC Dorschfontein.
3384 monticola var. monticola 3323 CA E Uniondale.
3398 arachnoidea var. namaquensis 3119 AB Koringberg.
3423 arachnoidea var. namaquensis 3119 BC Beeswater.
3439 floribunda var. dentata 3420 BA Bontebok Park.
3453 venosa ssp. venosa 3420 AB Bontebok Park.
3586 chloracantha var. denticulifera 3421 BB Cooper Siding.
3586 chloracantha var. denticulifera 3421 BD N Gouritzmond.
3612 arachnoidea var. nigricans 3320 AB Jagerskraal.
3620 marumiana var. viridis 3322 AC S Prince Albert.
3637 nortieri var. nortieri 3118 DC W Doornriver.
3670 marumiana var. viridis 3322 AC Prince Albert.
3906 nortieri var. pehlemanniae 3320 BB SW Laingsburg.
4160 bolusii var. bolusii 3323 BB NE Fullarton.
4168b glauca var. glauca 3324 AA E Humefield.
4179 glauca var. glauca 3224 CD Fairview.
4180 decipiens var. cyanea 3224 CD Fairview, W Jansenville.
4198 cooperi var. viridis 3325 AC Brakfontein.
4294 nigra var. diversifolia 3222 AC Wolwehoek.
4320 arachnoidea var. namaquensis 3220 DA Verlatenkloof.
4404 cooperi var. gordoniana 3323 CA Uniondale Poort.
4428 blackburniae var. blackburniae 3321 DA Rooiberg.
4429 blackburniae var. blackburniae 3321 DA Assegaaibos.
4430 herbacea var. paynei 3319 DD Olifantsdoorn.
4432 minima var. minima 3420 AB Bontebok Park.
4437 maraisii var. maraisii 3319 DD W McGregor.
4438 mucronata var. inconfluens 3320 DA Jakkalsfontein.
4439 herbacea var. herbacea 3319 CD N Lemoenpoort.
4440 decipiens var. cyanea 3322 BC E Klaarstroom.
4450 cooperi var. pilifera 3326 AD NW Salem.
4460 cooperi var. pilifera 3227 DC Brigadoon.
4461 maculata var. intermedia 3319 DC Buitenstekloof.
4462 arachnodea var. aranea 3322 AC S Cango Caves.
4462 arachnoidea var. scabrispina 3320 CA 20km W Ladismith.
4471 turgida var. suberecta 3421 BA Weltevrede.
4473 mutica var. mutica 3420 AC Soesriver.
4474 cooperi var. gordoniana 3324 DD NE Hankey.
4475 cooperi var. isabellae 3324 DD NE Hankey.
4476 cooperi var. isabellae 3324 DD E Hankey.
4476 turgida var. suberecta 3421 BA Draaihoek.
4477 turgida var. suberecta 3421 BA E Valsch River.
4478 turgida var. suberecta 3421 BB Gouritz River.
4479 mutica var. mutica 3419 BB E Riviersonderend.
4479 turgida var. longibracteata 3421 AB Kafferkuils Bridge.
4499 arachnoidea var. nigricans 3322 AC N Oudtshoorn.
4549 cymbiformis var. cymbiformis 3325 BC Kranspoort, W Patterson.
4575 arachnoidea var. nigricans 3320 DD W Warmwaterberg.
4642 mirabilis var. triebneriana 3419 BD Skietpad.
4647 arachnoidea var. aranea 3322 AD Raubenheimer Dam.
4648 cymbiformis var. ramosa 3327 AB Wooldridge.
4648 cymbiformis var. cymbiformis 3327 AB W Woolridge.
4649 cymbiformis var. reddii 3226 BB Klipplaat Dam.
4650 cymbiformis var. obtusa 3326 AC S Alicedale.
4651 cymbiformis var. obtusa 3226 DA Blinkwater.
4652 cymbiformis var. cymbiformis 3326 BB Ballinafad.
4653 cymbiformis var. obtusa 3325 BB Swartwaterpoort.
4654 cymbiformis var. cymbiformis 3327 AC Kapp‑Fish.
4655 cymbiformis var. cymbiformis 3226 DC Sulphur Baths.
4656 arachnoidea var. nigricans 3322 CA Volmoed.
4657 decipiens var. cyanea 3324 BD Hanekam.
4659 reticulata var. hurlingii 3320 CC Goudmyn.
4665 reticulata var. attenuata 3320 CC SE Bonnievale.
4665 reticulata var. reticulata 3319 DD Wolfkloof, Robertson.
4667 venosa ssp. tessellata 3225 BA Halesowen.
4668 venosa ssp. granulata 3320 AC Avondrus.
4669 venosa ssp granulata 3219 DC Skitterykloof.
4671 arachnoidea var. nigricans 3320 DC E Barrydale.
4677 venosa ssp. tessellata 3123 CD Nuwerus.
4677 heidelbergensis var. scabra 3420 AA Kliphoogte.
4901 marginata 3420 BA Koppies.
4902 serrata 3420 BA Koppies.
4941 sordida var. sordida 3325 AD SE Kirkwood.
5086 semiviva 3120 DB S Williston.
5096 semiviva 3120 DC S Williston.
5101 heidelbergensis var. scabra 3420 AD Haarwegskloof.
5157 decipiens var. decipiens 3322 AB Kleinsleutelftn.
5182 decipiens var. decipiens 3322 AA W Prince Albert.
5209 marumiana var. marumiana 3221 DD Tierberg.
5225 scabra var. scabra 3322 AC Cango.
5226 scabra var. scabra 3322 AC Cango.
5261 decipiens var. decipiens 3322 CA S Prince Albert.
5296 mucronata var. inconfluens 3320 CB Touwsfontein.
5750 venosa ssp. granulata 3220 CC Bantamsfontein.
5753 arachnoidea var. setata 3321 DA E Vanwyksdorp.
6486 arachnoidea var. scabrispina 3320 BB SW Laingsburg.
6488 venosa ssp. tessellata 3120 BC Beaufort West Dam.
6488 semiviva 3120 BC Beaufort West.
6490 venosa ssp. tessellata 3222 BA Karoo Nat.Pk.
6495 nigra var. nigra 3221 CB E Merweville.
6496 nigra var. nigra 3221 CA W Merweville.
6497 decipiens var. cyanea 3221 CB 15km Merweville to Koup.
6498 marumiana var. marumiana 3222 BA Molteno Pass.
6501 semiviva 3221 CA Langberg.
6502 nigra var. nigra 3221 CA Langberg.
6505 nortieri var. nortieri 3219 AC N Dwarsrivier.
6506 nortieri var. nortieri 3219 AD Dwarsrivier.
6509 maraisii var. maraisii 3320 CC W Bonnievale.
6510 maraisii var. maraisii 3320 CC W Bonnievale.
6511 maraisii var. maraisii 3319 DD Olifantskrans.
6512 mutica var. mutica 3420 AA SE Drew.
6512a maraisii var.. maraisii 3420 AA SE Drew.
6513 mirabilis var. triebneriana 3420 BD SW Swellendam.
6514 maculata var. intermedia 3319 DD Buitenstekloof.
6515 reticulata var. acuminata 3320 CC Aurora.
6516 maraisii var. meiringii 3319 DD Rooiberg, Goudmyn.
6518 maraisii var. maraisii 3319 DD Lower N Slopes, Rooiberg.
6519 maraisii var. maraisii 3319 DD Agter Vink.
6520 maraisii var. maraisii 3319 DD Die Nekkie, W Robertson.
6521 maraisii var. maraisii 3420 AA N Stormsvlei.
6522 arachnoidea var. arachnoidea 3319 DD Buitenstekloof.
6523 venosa ssp. venosa 3420 AB Swellendam.
6524 limifolia var. limifolia – – Komatipoort.
6525 rossouwii var. calcarea 3420 AD De Hoop.
6527 variegata var. hemicrypta 3420 AB NW Swellendam.
6528 floribunda var. floribunda 3420 AB SW Swellendam.
6533 maraisii var. maraisii 3420 AB N Napky.
6534 maraisii var. maraisii 3420 AA SE Drew.
6535 reticulata var. reticulata 3320 CC N slopes Rooiberg.
6536 mutica var. mutica 3420 AB S Napky.
6537 mirabilis var. triebneriana 3419 BC NE Goudini.
6538 mirabilis var. triebneriana 3419 BD 10km W Napier.
6539 maraisii var. maraisii 3420 AD Tarentaal.
6540 minima var. minima 3420 AD Tarentaal.
6541 heidelbergensis var. scabra 3420 AD NE Kathoek.
6542 variegata var. modesta 3420 BC E Potberg reidence.
6544 heidelbergensis var. scabra 3420 BC NN Potberg.
6545 heidelbergensis var. scabra 3420 BC N Potberg.
6546 heidelbergensis var. scabra 3420 AD NW Kathoek
6548 reticulata 3319 DD Bosfontein.
6551 floribundaXpygmaea 3421 BB NE Cooper siding.
6552 fasciata 3324 DD NE Zuurbron.
6553 cooperi var. gordoniana 3324 DD N Zuurbron.
6554 cooperi var. gordoniana 3324 DD N Zuurbron.
6555 cooperi var. isabellae 3325 CC Longmore Forest.
6556 bolusii var. pringlei 3225 DD NW Rippon Stn.
6557 cooperi var. pilifera 3325 BB NW Ripppon Stn.
6558 cooperi var. dielsiana 3225 DB N Eastpoort.
6559 cooperi var. dielsiana 3225 DB SE Eastpoort.
6560 cooperi var. dielsiana 3225 DB S Eastpoort.
6561 bolusii var. pringlei 3225 DB Baviaanskranz, Patryshoogte.
6562 cymbiformis var. obtusa 3226 CD Kagasmond.
6563 cooperi var. cooperi 3226 CD Koonap Bridge.
6564 cooperi var. dielsiana 3226 CB Chancery Hall.
6565 cooperi var. dielsiana 3225 DA W Somerset East.
6566 bolusii var. bolusii 3225 AC NE Ashbourne.
6567 cymbiformis var. reddii 3226 BB Waterdown Dam.
6568 nigra var. nigra 3225 BD Waterdown Dam.
6569 bolusii var. blackbeardiana 3226 BD Waterdown Dam.
6570 bolusii var. blackbeardiana 3226 BD S Estrelle.
6571 bolusii var. blackbeardiana 3227 AB Turnstream.
6572 cymbiformis var. reddii 3227 AB Turnstream.
6573 cymbiformis var. setulifera 3227 AB Highclere.
6574 nigra var. nigra 3224 DA 5km ENE Kendrew.
6575 nigra var. nigra 3226 CB Adelaide.
6580 decipiens var. virella 3224 DC Meerlust.
6581 decipiens var. virella 3224 DC Welgelegen.
6582 decipiens var. virella 3324 AB SW Mt Steward.
6583 decipiens var. virella 3324 AB NW Waaipoort.
6584 glauca var. herrei 3324 AD Waaipoort.
6584a glaucaXviscosa 3324 AD Waaipoort.
6585 zantneriana var. zantneriana 3324 AD Waaipoort.
6586 glauca var. herrei 3324 BC Zeekoeisnek.
6587 decipiens var. minor 3324 BC NW Die Bordjie.
6588 sordida var. lavranii 3324 BC NE DieBordjie.
6589 cooperi var. viridis 3324 BC NE Dorschfontein.
6591 cooperi var. pilifera 3226 DC S The Tower.
6592 cooperi var. dielsiana 3226 DC W Fort Beaufort.
6593 cymbiformis var. cymbiformis 3226 DC E The Tower.
6596 kingiana 3322 CC Moeras River.
6597 cooperi var. gordoniana 3424 BB Jeffrey’s Bay.
6598 glauca var. glauca 3325 CB Bauerskraal.
6599 cooperi var. pilifera 3325 CB Bauerskraal.
6600 cooperi var. viridis 3325 AC N Perdepoort.
6601 arachnoidea var. aranea 3322 CC Moeras River.
6602 cooperi var. pilifera 3326 BC Glen Craig.
6603 cooperi var. gracilis 3326 BA NE Grahamstown.
6604 decipiens var. xiphiophylla 3325 DC Coega.
6608 arachnoidea var. setata 3322 CB N Dysselsdorp.
6609 truncata 3322 CB N Dysseldorp.
6610 arachnoidea var. setata 3324 AC N Steytlerville.
6611 sordida var. sordida 3325 DA Soutkloof.
6612 aristata 3325 DA Soutkloof.
6613 sordida var. sordida 3325 BC Bluecliff Stn.
6614 cooperi var. gracilis 3326 AB Hellspoort.
6615 glauca var. glauca 3325 AC Paardepoort.
6616 decipiens var. xiphiophylla 3325 CA Bauerskraal.
6618 decipiens var. minor 3325 AC Sapkamma/Perdepoort.
6619 decipiens var. minor 3325 AC Sapkamma/Perdepoort.
6620 decipiens var. minor 3325 AC Sapkamma.
6621 outeniquensis 3322 CC Moerasriver.
6622 pumila 3319 DA Mowers.
6623 pumila 3319 DC W Rooiberg.
6624 minima var. poellnitziana 3320 CC W Sanddrift.
6625 maraisii var. maraisii 3320 CC Sanddrift.
6626 heidelbergensis var. scabra 3320 CC N Sanddrift.
6627 marginata 3320 CC N Sanddrift.
6628 minima var. poellnitziana 3320 CC Sanddrift.
6629 marginataXminima 3320 CC E Sanddrift.
6630 minima var. minima 3419 DB W Moddervlei, Elim.
6631 mirabilis var. mirabilis 3419 DB Mierkraal.
6632 rossouwii var. calcarea 3420 CA Renosterfontein.
6633 marginata 3420 AC Adoonskop.
6634 marginata 3420 AC Adoonskop.
6635 mirabilis var. badia 3419 BD Napier.
6636 mirabilis var. triebneriana 3419 BA S Greyton.
6637 minima var. minima 3419 DB Mierkraal.
6638 maraisii var. maraisii 3420 AC Rooivlei, Bredasdorp.
6639 mirabilis var. sublineata 3420 CA S Bredasdorp.
6640 maraisii var. maraisii 3420 AC Adoonskop.
6641 mutica var. mutica 3420 AC Hasiesdrift.
6642 pumila 3419 DD Vinkrivier.
6643 mirabilis var. triebneriana 3419 BD Fairfield.
6644 mirabilis var. triebneriana 3420 BD SW Swellendam.
6645 pumila 3319 CB Worcester airfield.
6646 maraisii var. maraisii 3319 DD N Macgregor.
6647 maraisii var. maraisii 3319 DD Agter Vink.
6648 maraisii var. maraisii 3319 DD SW Robertson.
6649 arachnoidea var. setata 3320 CC E Montagu.
6650 mutica var. nitida 3420 BB SE Heidelberg.
6651 magnifica var. magnifica 3421 AA S Riversdale.
6653 emelyae var. emelyae 3321 CD N Sandkraal.
6654 emelyae var. emelyae 3321 CD N Sandkraal.
6655 emelyae var. emelyae 3321 CD N Sandkraal.
6658 arachnoidea var. aranea 3321 CD E Sandkraal.
6659 emelyae var. emelyae 3321 CD SE Vanwyksdorp.
6660 emelyae var. multifolia 3321 CC W Muiskraal.
6661 arachnoidea var. nigricans 3321 CC W Muiskraal.
6662 magnifica var. atrofusca 3421 AA Kweekkraal.
6663 magnifica var. magnifica 3420 BB SW Heidelberg.
6666 magnifica var. magnifica 3420 BA S Tradouw Pass.
6667 maraisii var. maraisii 3320 DC SW Barrydale.
6668 maraisii var. maraisii 3320 CC N Ashton.
6670 maraisii var. maraisii 3319 DD W Robertson.
6672 maraisii var. maraisii 3319 DD Koningsriver.
6673 maraisii var. maraisii 3319 DD Kranz Reserve.
6674 pumila 3319 DD Kranz Reserve.
6676 maraisii var. meiringii 3320 CC E Goudmyn.
6678 maraisii var. maraisii 3319 DD Grootrivier.
6680 herbacea var. paynei 3319 DD Koningsriverberg.
6681 maraisii var. maraisii 3319 DD Koningriver Dam.
6682 maraisii var. maraisii 3319 DD N Koningriver Dam.
6683 maraisii var. notabilis 3319 DD N Klaasvoogds.
6684 reticulata var. attenuata 3320 CC E Dankbaar.
6685 mirabilis var. diversicolor 3320 BB Olifantsdoornkloof.
6686 mirabilis/maraisii 3320 BB E Olifantsdoornkloof.
6687 maraisii var. maraisii 3319 DD SE McGregor.
6688 herbacea var. herbacea 3319 DA Mowers.
6690 arachnoidea/mucronata 3320 CA Watervalkloof.
6691 maraisii var. maraisii 3319 DC NW Boschfontein.
6692 reticulata var. reticulata 3319 DC NW Boschfontein.
6693 reticulata var. subregularis 3319 DC Uitvlug.
6694 arachnoidea var. arachnoidea 3319 DA Kanetvlei.
6696 arachnoidea var. arachnoidea 3319 BD W Osplaas.
6697 arachnoidea var. arachnoidea 3319 BD E Osplaas.
6698 venosa ssp. granulata 3319 BA Karoopoort.
6700 arachnoidea var. arachnoidea 3320 CA Soutkuil.
6702 pulchella var. pulchella 3320 CA Soutkuil.
6703 arachnoidea var. arachnoidea 3320 DA Bellair Dam.
6704 arachnoidea/mucronata 3320 CB Ouberg.
6705 arachnoidea/mucronata 3320 CB Ouberg.
6706 arachnoidea/mucronata 3320 CB Ouberg.
6710 mirabilis var. depauperata 3420 BB Boesmansrivier.
6711 mirabilis var. depauperata 3420 BB Boesmansrivier.
6712 mirabilis var. depauperata 3420 BB Boesmansrivier.
6713 mirabilis var. depauperata 3420 BB Boesmansrivier.
6714 mirabilis var. depauperata 3319 DC Koeniesriver.
6719 pumila 3319 DD Dassiehoek.
6720 mirabilis var. depauperata 3319 DC Rietvleikloof.
6721 reticulata var. reticulata 3319 DD W Robertson.
6722 pumila 3319 DC Boschfontein.
6723 mirabilis var. depauperata 3319 DC Takkap.
6724 emelyae var. emelyae 3322 CD Klipdrif.
6725 pungens 3323 DD W Braamrivier.
6727 outeniquensis 3322 CD Herold.
6727 scabra var. scabra 3323 DB Braamrivier.
6728 scabra var. scabra 3323 CC W DeVlugt.
6729 transiens 3323 CC N De Vlugt.
6730 mucronata var. habdomadis 3321 AD Seweweekspoort.
6732 scabra var. starkiana 3322 AD Lentelus.
6737 reticulata var. reticulata 3319 DC N Mowers Stn.
6738 arachnoidea var. arachnoidea 3319 DC N Mowers Stn.
6739 arachnoidea var. nigricans 3321 CC W Springfontein.
6740 arachnoidea var. aranea 3320 DD Kleindoringrivier.
6741 emelyae var. major 3320 DD Kleindoringrivier.
6743 mucronata var. mucronata 3320 DD SSE Barrydale.
6744 arachnoidea var. setata 3320 DC Tradouw Pass.
6745 floribunda var. floribunda 3421 AB RoseInnis Drive.
6746 chloracantha var. denticulifera 3321 BB NW Herbertsdale.
6747 magnifica var. acuminata 3421 BD Vleesbaai.
6749 turgida var. suberecta 3421 BB Die Hoek, Gouritz.
6750 minima var. minima 3421 BD Melkhoutfontein.
6751 magnifica var. splendens 3421 AB Soutpan, W Albertinia.
6756 herbacea var. paynei 3319 DD S Olifantsdoorn.
6757 mirabilis var. depauperata 3419 BB Hoeksrivierkloof.
6758 nortieri var. nortieri 3219 CD 5km SE Sneeukop.
6759 nortieri var. nortieri 3219 CD 10km SE Sneeukop.
6760 maraisii var. maraisii 3319 DD Houtbaaiskloof.
6761 maraisii var. meiringii 3319 DD Top Bakenskop.
6762 arachnoidea var. setata 3320 DB Kuitfontein, NE Plathuis.
6763 pulchella var. globifera 3320 DB Wolwefontein, Plathuis.
6764 blackburniae var. blackburiae 3320 DB Wolwefontein.
6767 outeniquensis 3322 CC Herold.
6768 fasciata 3324 CD Moordenaarskloof.
6769 viscosa 3324 CD Moordenaarskloof.
6770 viscosaXfasciata 3324 CD Moordenaarskloof.
6771 transiens 3324 CD Moordenaarskloof.
6772 pungens 3324 CC Joubertskraal.
6773 cooperi var. isabellae 3324 CC Joubertskraal.
6774 scabra var. scabra 3323 DD Braamrivier.
6775 scabraXpungens 3323 DD Braamrivier.
6776 cooperi var. cooperi 3225 DA SW Glen Avon.
6777 nigra var. nigra 3225 DA N Slagtersnek.
6778 cooperi var. pilifera 3225 DD New Slagtersnek.
6780 pungens 3323 DD W Braamrivier.
6781 maraisii var. notabilis 3319 DD Bergplaas.
6783 mirabilis var. mirabilis 3419 DB Mierkraal.
6784 cooperi var. gordoniana 3324 DC Draaihoek.
6785 cooperi var. gordoniana 3324 DC Draaihoek.
6788 viscosa 3324 DA Komdomo.
6789 cooperi var. picturata 3324 DA N Komdomo
6790 cooperi var. picturata 3324 DC W Kranz, E Wistaria.
6791 cooperi var. picturata 3324 DC E Kranz, E Wistaria.
6792 cooperi var. gordoniana 3424 CB N Jeffrey’s Bay.
6793 cooperi var. gordoniana 3324 DD N Zuurbron.
6794 fasciata 3324 DD Hankey Pass.
6795 fasciata 3424 BB Kabeljouws.
6796 fasciata 3424 BB N Jeffrey’s Bay.
6797 fasciata 3424 BB W Bank Zeekoevlei.
6798 gracilis var. isabellae 3324 DB Houtkloof, Elandsriver.
6799 cooperi var. gordoniana 3324 DD E Roodewal.
6801 gracilis var. isabellae 3324 DD Milton, SW Hankey.
6802 gracilis var. isabellae 3324 DD Philips Tunnel.
6803 attenuata var. radula 3324 DD Hallelujah, N Hankey.
6804 cooperi var. gordoniana 3324 DD NE Hankey.
6805 cooperi var. gordoniana 3324 DD Hallelujah.
6806 fasciata 3325 CC Loerie Dam.
6807 fasciata 3324 DD E Hankey.
6808 cooperi var. isabellae 3325 CC E Gamtoos Bridge.
6809 fasciata 3325 CC S Gamtoos Bridge.
6810 cooperi var. gordoniana 3323 DD N Joubertina.
6811 cooperi var. gordoniana 3323 CA Uniondale Poort.
6813 pygmaea var. pygmaea 3422 AA Moss Gas, W Mossel Bay.
6815 maculata var. maculata 3319 CB Audensberg Peak.
6816 sordida var. sordida 3325 BC SE Kirkwood.
6817 magnifica var. atrofusca 3421 AA NW Kweekkraal.
6820 monticola var. monticola 3322 DB W Uniondale, NE Kykoe.
6821 scabra var. scabra 3322 DB NE Kykoe.
6822 viscosa 3323 DA Bottom Nuwekloof.
6823 monticola var. bavensis 3324 CA Top Bosrug.
6824 cooperi var. isabellae 3324 CA Koutnek.
6825 transiens 3324 CA S Geelhoutboskloof.
6826 cooperi var. isabellae 3324 CA Rooikloof.
6827 cooperi var. picturata 3324 CB E Rooihoek.
6828 attenuata 3324 DA Sandlands Cambria.
6830 cooperi var. gordoniana 3324 DA E Komdomo.
6831 attenuata var. attenuata 3324 DD Hallelujah, N Hankey.
6832 cooperi var. tenera 3326 BA E ft Plutosvale.
6833 cooperi var. tenera 3326 BA ft Plutosvale.
6834 cooperi var. tenera 3326 BA W ft Plutosvale.
6835 cooperi var. tenera 3326 BA WW ft Plutosvale.
6836 cymbiformis var. incurvula 3326 BA W Roffs Rock, Plutosvale.
6837 cymbiformis var. incurvula 3326 BA S Roffs Rock, Plutosvale.
6838 cymbiformis var. incurvula 3326 BA SE Roffs Rock, Plutosvale.
6839 cymbiformis var. incurvula 3326 BA W Mindmill, Plutosvale.
6840 cooperi var. tenera 3326 BA opposite windmill, mid Plutosvale.
6841 attenuata 3326 BA Roffs Rock, Plutosvale.
6842 bolusii var. blackbeardiana 3227 BC Inverbolo.
6843 cymbiformis var. reddii 3227 BC Inverbolo.
6844 angustifolia 3326 AC E Alicedale.
6845 cooperi var. cooperi 3326 AC E Alicedale.
6847 cymbiformis var. obtusa 3326 AC SW Alicedale.
6848 cymbiformis var. obtusa 3326 AC NW Alicedale.
6849 angustifolia 3325 BB Aalwynspoort. Stn.
6850 cymbiformis var. obtusa 3325 BB Swartwaterpoort.
6851 aristata 3325 BB Modderfontein, S Verdun.
6852 aristata 3325 BA Stonefountain.
6855 decipiens var. virella 3325 AA E Waterford.
6856 bolusii var. bolusii 3323 BB NE Fullarton.
6858 glauca var. herrei 3323 BB NE Fullarton.
6859 floribunda var. major 3420 AB SW Swellendam.
6860 maraisii/mirabilis 3420 AB 4km SW Swellendam.
6861 maraisii/heidelbergensis 3420 AB SW Swellendam.
6862 mirabilis/maraisii 3420 AA NW Rondeheuwel.
6863 mirabilis var. diversicolor 3419 BB Boesmansriver/Olifantsdoornkloof.
6864 mirabilis var. depauperata 3319 DD Whipstock, W McGregor.
6865 mirabilis var. consanguinea 3319 DD Dwarswaterkloof, W McGregor.
6867 arachnoidea var. arachnoidea 3320 CB NE Ouberg.
6868 arachnoidea var. arachnoidea 3320 CD W Twistniet.
6871 arachnoidea var. arachnoidea 3319 DD Pietersfontein. M
6873 arachnoidea var. arachnoidea 3319 DD NW Pietersfontein.
6875 maraisii var. maraisii 3320 CC NE Ashton, Cogmanskloof.
6876 mirabilis var. depauperata 3419 BB E Olifantsdoornkloof.
6877 maraisii var. maraisii 3319 DD Die Nekkie.
6878 maraisii/mirabilis 3419 BB SW Swellendam.
6879 maraisii/floribunda 3420 BA Koppies. SE Swellendam.
6880 rossouwii var. rossouwii 3420 BA Oudekraalkop.
6881 floribunda var. dentata 3420 BA S Oudekraalkop.
6882 heidlbergensis 3420 AD W Juliesfontein.
6883 mucronata var. mucronata 3320 CD Poortjieskloof.
6884 cymbiformis var. incurvula 3326 BA Roff’s Rock, N Plutosvale.
6885 decipiens var. cyanea 3222 DC Trakaskuilen.
6886 heidelbergensis/mirabilis 3420 AD N Juliesfnt.
6888 variegata var. modesta 3420 BC NW Slopes Potberg.
6889 heidelbergensis var. scabra 3420 BC NW slopes Potberg.
6890 heidelbergensis var. scabra 3420 AD Witkop, N Brakfontein.
6891 cooperi var. tenera 3326 BA 300m W lower Roff’s Rock
6892 cymbiformis var. incurvula 3326 BA below 6891, Roff’s Rock.
6893 cymbiformis var. incurvula 3326 BA 400m W Roff’s Rock.
6894 angustifolia 3326 AB Thornkloof, NW Grahamstown.
6895 cymbiformis var. obtusa 3326 AB SE Thornkloof.
6896 coarctata var. adelaidensis 3326 AB SE Thornkloof.
6897 aristata 3325 BB SE Commadagga.
6899 aristata 3325 AB Paddafontein.
6901 aristata 3325 AB W Hopewell.
6903 cymbiformis var. cymbiformis 3325 AD S Spekboomkop.
6904 cooperi var. gordoniana 3325 AD Top Wilgerfontein
6905 cooperi var. “puberula” 3325 AD Top Wilgerfontein.
6907 glauca/coarctata 3325 AD Top Wilgerfontein.
6908 glauca 3325 AD SE DePlaat.
6909 cooperi var. gracilis 3325 AD SE DePlaat.
6910 cooperi var. “puberula” 3325 AD SE DePlaat.
6911 cooperi var. gracilis 3325 AD Kaboegapoort.
6914 cooperi var. gracilis 3325 AD E Spekboomberg.
6915 cooperi var. gracilis 3325 AD SE Koksedam
6916 aristata 3325 AB Spitzkop, Kaboega.
6917 aristata 3325 AB Original Kaboega gate.
6920 aristata 3325 DA Soutkloof.
6921 cooperi var. doldii 3327 BA Chalumna.
6922 cooperi var. pilifera 3325 BA Oudekraal.
6924 cooperi var. gracilis 3325 AD Kaboega Dam.
6925 cymbiformis var. cymbiformis 3325 AD Koksepad.
6927 bolusii var. pringlei 3325 BB W Ripon Stn.
6928 cooperi var. isabellae 3324 DC Nuwelande, Patensie.
6929 longiana 3324 DC Nuwelande, Patensie.
6930 cooperi var. picturata 3324 DC E Andrieskraal.
6932 cooperi var. isabellae 3424 BB Krom River, Ripon.
6933 cooperi var. gordoniana 3424 BB E Humansdorp.
6934 fasciata 3424 BB SW Humansdorp.
6935 cooperi var. gracilis 3325 AD S DePlaat.
6936 cooperi var. gordoniana 3323 CA N Uniondale.
6937 decipiens var. scottiana 3323 CA Vetvlei.
6938 decipiens var. scottiana 3323 CA Woedjieskloof, NE Uniondale.
6939 decipiens var. scottiana 3323 AD Ghwarriepoort, Keurfontein.
6940 cooperi var. gracilis 3325 AB Klipfontein, E DePlaat.
6942 aristata 3325 AB DePlaat Weir.
6943 cooperi var. gracilis 3325 AD Dassiekop.
6945 nigra 3325 AB N DePlaat house.
6947 sordida 3325 AD S DePlaat house.
6949 cooperi var. pilifera 3325 AD Vyeboomfontein.
6951 maraisii var. maraisii 3320 CC NN Ashton.
6952 marginata 3320 CC N Ashton.
6953 maraisii var. maraisii 3319 DD Le Chasseur Nek.
6954 maraisii var. maraisii 3420 AA N Stormsvlei.
6955 mirabilis var. triebneriana 3420 AA Stormsvlei.
6956 maraisii var. maraisii 3319 DD SW Rooiberg, Eilandia.
6957 reticulata 3319 DD S Rooiberg.
6958 pumila 3319 DD S Rooiberg.
6959 maraisii var. maraisii 3319 DD Upper SE Rooiberg.
6959 maraisii var. maraisii 3319 DD Kleinspitzkop, Vrolikheid.
6960 maraisii var. maraisii 3320 CC W Sanddrift.
6961 maraisii var. maraisii 3319 DD Muiskraalkop.
6962 maraisii var. maraisii 3320 CC Olifantskrans.
6963 maraisii var. maraisii 3320 CC W Bonnievale.
6964 maraisii var. maraisii 3320 CC E Zandvliet.
6965 maraisii var. maraisii 3320 CC far E Zandvliet.
6966 maraisii var. maraisii 3319 DD Steenbokshoogte, Vrolikheid.
6967 maraisii var. maraisii 3319 DD W Kanonkop, Vrolikheid.
6968 maraisii var. maraisii 3319 DD Witkranz, Vrolikheid.
6969 maraisii var. maraisii 3319 DD Kleinspitzkop, Vrolikheid.
6970 herbacea var. flaccida 3319 DD Rooiberg, Vinkrivier.
6971 heidelbergensis/maraisii 3320 CC Jakkalshoek, E Bonnievale.
6972 maraisii var. maraisii 3319 DD with flaccida, Rooiberg.
6973 maraisii var. maraisii 3419 BD N Napier.
6974 heidelbergensis var. scabra 3320 CC Tonteldooskop.
6976 heidelbergensis var. scabra 3320 CC S Tonteldooskop.
6977 heidelbergensis var. scabra 3320 CC S aspect at 6974.
6978 heidelbergensis var. scabra 3320 CC between 6974/6977.
6979 heidelbergensis/maraisii 3320 CC Loerkop, E. Ashton.
6981 heidelbergensis/maraisii 3320 CC SE Loerkop.
6982 mutica var. mutica 3420 AC Hasiesdrift.
6983 rossouwii var. rossouwii 3420 CA Soutkloof, NW Bredsadorp.
6984 rossouwii var. rossouwii 3420 CA Nooitgedacht.
6985 rossouwii var. calcarea 3420 AD DeHoop.
6986 rossouwii var. petrophila 3420 CA Karsriver.
6987 mirabilis var. badia 3419 BD Sandfontein.
6988 pumila 3319 DD Pietersfontein.
6988 heidelbergensis var. scabra 3320 CC Nooitgedacht.
6989 heidelbergensis var. scabra 3320 CC SE Nooitgedacht.
6990 heidelbergensis var. scabra 3320 CC NE Mardouw.
6991 mucronata var. inconfluens 3320 DA Jakkalsfontein.
6992 heidelbergensis var. scabra 3320 CD Langverwacht.
6994 arachnoidea var. nigricans 3321 DB NE Tierkloof, Gamka.
6995 viscosa 3321 DB E Tierkloof.
6996 arachnoidea var. setata 3321 DB SE Tierkloof.
6997 viscosa 3321 DB S Tierkloof.
6998 arachnoidea var. setata 3321 DA Top Rooiberg.
7001 arachnoidea var. setata 3321 DA Groenefontein.
7002 viscosa 3321 DA Groenefontein.
7003 arachnoidea var. setata 3321 DA W Groenefontein.
7006 bolusii var. pringlei 3225 CC Moederzoonpoort.
7008 bolusii var. pringlei 3225 CC Rietvlei, NE Waterford.
7010 aristata 3325 AB SE DePlaat Weir.
7011 aristata 3325 AB SE DePlaat Weir.
7012 aristata 3325 AB Buffelsnek, Kaboega.
7014 truncata 3322 CB N Dysseldorp.
7017 cooperi var. “puberula” 3325 BC S Klipfontein.
7021 bolusii var. bolusii 3225 CC SE Pearston.
7022 decipiens var. virella 3225 CC SE Pearston.
7023 decipiens var. virella 3224 DB Ebenezer.
7024 viscosa 3322 AB Sleutelfontein.
7025 decipiens var. cyanea 3222 DC Trakaskuilen.
7026 decipiens var. decipiens 3322 BC Klaarstroom.
7028 decipiens var. xiphiophylla 3325 AA Darlington Dam.
7029 mutica var. mutica 3420 AD SW Swellendam.
7030 maraisii var. maraisii 3420 AB Napky, SW Swellendam.
7031 mutica var. mutica 3420 AB N Napky.
7032 mutica var. mutica 3420 AA Rietfontein.
7033 arachnoidea var. arachnoidea 3319 DD W Cape Lime.
7035 kingiana/minima 3321 AD Rooiberg, S Calitzdorp.
7036 heidelbergensis var. scabra 3320 CC Witkop, SW Janharmsgat.
7037 heidelbergensis var. scabra 3320 CC Middelrivier, Drew.
7038 marginataXpumila 3320 CC NW Sanddrift.
7041 decipiens var. virella 3224 DD Palmietfontein, NE Jansenville.
7042 bolusii var. bolusii 3224 DD Hinchenbrook.
7043 decipiens var. virella 3224 DD Langollen.
7046 bolusii var. bolusii 3224 DD DeRust, S Lootskloof.
7047 decipiens var. virella 3224 DD DeRust, S Lootskloof.
7049 cooperi var. pilifera 3325 AD SE Klipfontein, Kaboega.
7051 cooperi var. gracilis 3326 AB Helspoort, upper W.
7052 cooperi var. gracilis 3326 AB Helspoort, lower E.
7053 cooperi var. gracilis 3326 AB Helspoort, lower W.
7054 cooperi var. gracilis 3326 AB Helspoort, upper E.
7055 maraisii var. nov. 3420 CC Rietvlei (Shielam).
7056 heidelbergensis var. scabra 3420 CC NW Sandrift.
7057 heidelbergensis var. scabra 3420 CC W Sanddrift.
7058 pumilaXmarginata 3420 CC S Sanddrift, Drew.
7059 mirabilis var. triebneriana 3419 BC Jongensklip.
7060 mutica var. mutica 3420 AD Beyersdal.
7061 variegata var. modesta 3420 AD Kathoek.
7063 heidelbergensis 3420 AD SE Beyersdal.
7064 mutica var. mutica 3320 CC Sanddrift.
7065 pubescens var. pubescens 3319 DC Sandberg.
7066 pubescens var. livida 3319 CD Lemoenpoort.
7067 herbacea 3319 DC Sandberg.
7068 arachnoidea var. scabrispina 3320 BB Witteberg Rd.
7069 viscosa 3320 BB Witteberg Rd.
7070 nortieri var. pehlemanniae 3320 BB NW Laingsburg.
7071 arachnoidea var. scabrispina 3320 BB NW Laingsburg.
7072 marumiana var. dimorpha 3320 AD Konstabel.
7073 wittebergensis 3320 BB S Laingsburg.
7074 mirabilis var. triebneriana 3419 BB Verdwaalskloof.
7075 mutica var. mutica 3420 DD Grootvlakte, E Riviersonderend.
7076 herbacea var. paynei 3419 DD S Grootrivier.
7077 maraisii var. maraisii 3319 DD Wolwedrif.
7078 pumila 3319 DD Wolwedrif.
7079 maraisii var. meiringii 3319 DD W Middelplaas.
7080 maraisii var. meiringii 3320 CC W Wakkerstroom.
7081 maraisii var. meiringii 3320 CC E Goudmyn.
7082 maraisii var. meiringii 3320 CC Bobbejaanskloof.
7083 maraisii var. meiringii 3320 CC NE Goudmyn.
7084 maraisii var. meiringii 3320 CC E Olive grove.
7085 maraisii var. meiringii 3320 CC Station Hill.
7086 maraisii var. maraisii 3420 AA SE Bokdam.
7087 mirabilis var. triebneriana 3420 AA Brakfontein.
7088 reticulata var. reticulata 3319 DD W Middelplaas.
7089 pumila 3320 CC E Bonnievale.
7090 mirabilis var mirabilis 3419 DB Mierkraal.
7091 mirabilis var triebneriana 3419 BD NW Napier.
7092 mirabilis var triebneriana 3420 AB Elandskloof.
7096 pumila 3319 DD Koningsriver.
7097 maraisii var notabilis 3319 DD Kranzkop.
7098 maraisii var notabilis 3319 DD Bergplaas.
7099 heidelbergensis 3420 AA Nuutbegin.
7100 minima 3420 AA Nuutbegin.
7101 variegata 3321 AB Hoekraal.
7102 magnifica var splendens 3321 BA Welgevonden.
7103 floribunda var dentata 3321 BA N Ouvloer.
7104 floribunda var dentata 3321 BA NE Ouvloer.
7105 turgida var suberecta 3321 BA NE Ouvloer.
7106 floribunda var dentata 3321 AB Snymanskraal.
7107 retusa 3321 AA NE Melkboom.
7108 heidelbergensis 3321 AA NE Melkboom.
7109 heidelbergensis 3321 AA Klien Kragga.
7110 retusa 3321 AA N Melkboom.
7111 heidelbergensis 3321 AA N Melkboom.
7112 heidelbergensis 3321 AA NW Kweekkraal.
7113 heidelbergensis 3321 AA NW Kweekkraal.
7114 heidelbergensis 3321 AA NW Kweekkraal.
7115 heidelbergensis 3321 AA NW Kweekkraal.
7116 heidelbergensis 3321 AA NW Kweekkraal.
7117 heidelbergensis 3321 AA NW Kweekkraal.
7118 heidelbergensis 3321 AA NW Kweekkraal.
7121 cymbiformis 3325 BD Gatjewolf.
7125 attenuata 3325 BC N Enon.
7126 cooperi var puberula 3325 AD Wilgerfontein.
7127 glauca 3325 AD Buffelsnek.
7128 angustifolia var baylissii 3325 BD Wells Gate.
7130 venosa subsp granulata 3319 BA Karoopoort.
7131 venosa subsp granulata 3220 CC E Bizansgat.
7133 mutica var nigra 3420 BB Goedehoop.
7134 mutica var nigra 3420 BB Klipdrift.
7135 venosa subsp venosa 3420 BB Sandkraal.
7136 limifolia Bridgewater Nursery.
7137 limifolia 2732 CD Pumalanga.
7138 limifolia 2732 CB Inxwala, Mkhuze.
7139 limifolia 2731 BC Ncotshane, Pongola.
7140 limifolia 2731 BC Draaiwater, Pakamisa.
7141 limifolia 2531 CC N Rymers Creek.
7142 limifolia 2531 CC Mays Mine.
7143 limifolia 2531 CB Boondoks.
7144 limifolia 2531 CB N Manders Dam.
7145 limifolia 2531 CB Three Sisters.
7146 limifolia 2731 CA Bivaan Dam.
7147 limifolia 2731 CB Ithala.
7148 limifolia 2731 AD Ithala.
7149 viscosa N.Koup Stn.
7150 decipiens var cyanea W.Merweville.
7151 heidelbergensis 3421 AA N Kweekkraal W.
7152 heidelbergensis 3421 AA N Kweekkraal W.
7153 heidelbergensis 3421 AA N Kweekkraal W.
7154 heidelbergensis 3421 AA N Kweekkraal W.
7155 floribunda 3421 AA SW Pretoriuskop.
7156 floribunda 3421 AA W Pretoriuskop.
7157 atrofusca 3421 AA N Bosgasiekop.
7158 floribunda 3421 AA N Bosgasiekop.
7159 atrofusca/floribunda 3421 AA N Bosgasiekop.
7160 magnifica 3421 AA N Kweekkraal E.
7161 magnifica 3421 AA N Kweekkraal E.
7162 magnifica 3421 AA N Kweekkraal E.
7163 magnifica 3421 AA S Riversdale t.loc.
7164 floribunda cf. 3420 BD Dassieklip.
7165 heidelbergensis 3420 BB Matjestoon.
7166 pumila 3319 BD Mowers Stn.
7167 pumila 3319 BC Apiesklip.
7168 cooperi var 3325 AD Kabouga.
7169 attenuata 3325 BC Enon.
7170 cymbiformis var 3325 BC Enon.
7171 glauca/coarctata 3325 AD S Wilgefontein.
7172 floribunda 3421 AA N Kweekkraal E.
7173 retusa 3421 AA N Kweekkraal E.
7174 maraisii 3319 DD Skurweberg.
7175 pumila 3319 DD W Highlands, Goree.
7176 maraisii 3319 DD N Agtervink.
7178 viscosa 3323 BD Ruiterspoort.
7179 viscosa 3323 BD Constantia.
7180 decipiens 3323 BD Constantia.
7182 monticola 3323 BC SW Koegoeroe.
7183 monticola 3323 BC SE Koegoeroe.
7184 bayeri 3323 CA Uniondale Fort.
7185 monticola 3323 CA Uniondale Height.
7188 decipiens/scottiana 3323 CA 5km E Hoekplaas.
7189 decipiens/scottiana 3323 CA 2km NW Hoekplaas.
7190 mucronata 3322 BD 3km E Rooiloop Stn.
7193 cf scottiana 3322 BC 5km W Rooiloop Stn.
7194 arachnoidea 3322 DA E Middelplaas Stn.
7195 arachnoidea 3322 BC 2km E Vlakteplaas.
7196 bayeri 3322 BC SW Derust.
7200 truncata 3322 CA NE Oudtshoorn.
7201 truncata 3322 CA Volmoed.
7202 morrisiae 3322 CA Volmoed.
7203 arachnoidea 3321 CB Ladismith.
7204 scabra 3322 DA SE Doornkloof.
7205 arachnoidea var nigricans 3321 DA W Vensterkrans.
7206 arachnoidea var nigricans 3321 CA Buffelsdrif S.
7207 arachnoidea var nigricans 3321 CA Buffelsdrif.
7208 arachnoidea var nigricans 3321 CA Bakenskop.
7210 arachnoidea var nigricans 3321 CA E Vensterkrans.
7211 arachnoidea var nigricans 3321 CC W Springfontein.
7212 mucronata var mucronata 3321 CC Tradouwshoek.
7213 mucronata var mucronata 3321 CC S Barrydale S aspect.
7214 maraisii 3321 CC S Barrydale S aspect.
7215 mucronata var mucronata 3321 CC S Barrydale.
7216 mucronata var mucronata 3321 CC S Barrydale
7217 heidelbergensis/magnifica 3421 AA Rooikop Melkboom
7218 floribunda/magnifica 3420 BB Duiwenhoks Morn Star
7219 turgida 3420 BB Duiwenhoks Morn Star
7220 floribunda/magnifica 3420 BB NE Morning Star
7221 mutica var nigra 3420 BB NE Morning Star
7223 scabra 3322 DA Diepkloof, E De Rust
7224 floribunda/chloracantha 3421 BB Cooper Siding
7225 retusa 3421 AA S Droekloof Riversd.
7226 turgida 3420 BB Witheuwel Pienaarsr
7227 heidelbergensis/magnifica 3420 BB Witheuwel Pienaarsr
7228 turgida 3420 BB Somona S Heidelberg
7229 heidelbergensis/magnifica 3420 BB Somona S Heidelberg
7231 bayer1 (seed) 3322 DA “Kleingeluk, E DeRust”
7232 turgida/heidelbergenss 3420 BB S Heidelberg Reserve
7233 turgida/heidelbergenss 3420 BB Hooikraal (Die Plotte N)
7234 turgida/heidelbergenss 3420 BB Hooikraal (Die Plotte S)
7236 turgida/retusa 3420 BB E Heidelberg Dam
7237 magnifica cf.var atrofusca 3420 BB Andrieskraal/Skeideing
7238 magnifica cf.var atrofusca 3420 BB Andrieskraal/Skeiding
7239 magnifica cf.var atrofusca 3420 BB Andrieskraal/Skeiding
7240 turgida/retusa 3420 BB Skeiding
7241 maraisii 3419 DD Voordieberg, McGregor
7242 mirabilis/maraisii 3420 AA Witkop, N Uitvlug Annex
7243 mirabilis/maraisii 3420 AA SE Uitvlug Annex
7244 mirabilis/maraisii 3420 AB E Die Kop, S Uitvlug.
7245 mirabilis/maraisii 3420 AB do. above, E
7246 mutica 3420 AC Verbrandskloof
7247 mirabilis/maraisii 3419 BD 0.5km NE Napier
7248 mirabilis/maraisii 3420 DB N, Infanta
7249 mirabilis/maraisii 3420 DB N, Infanta
7250 maraisii/heidelbergensis 3420 BC Uitrus (Whisky Creek)
7251 mirabilis 3420 BC Sandhoogte, DeHoop
7252 mirabilis 3420 AA NE Klipfontein
7253 mirabilis 3420 AA N Klipfontein
7254 mirabilis 3420 AA NW Noukloof Homestead
7255 elizeae 3420 AA 28km E Riviersonderend
7257 maraisii 3420 AA SW Stormsvlei homestead
7258 minima 3420 BC Uitrus (Whisky Creek)
7259 mirabilis 3419 BD Napier Stn
7260 mirabilis 3419 BD S Mierkraal, Napier
7261 mirabilis 3420 AC NW Bredasdorp
7262 mirabilis 3420 BA Ouplaas, SE Greyton
7263 maraisii 3319 DC Droerivierberg B
7264 maraisii 3319 DC Droerivierberg A
7265 maraisii 3319 DC Haumanskloof
7266 pubescens var livida 3319 CD E Lemoenpoort S
7268 herbacea 3319 CD E Lemoenpoort S
7269 maraisii v meiringii 3320 CC Edendale, Bonnievale
7270 maculata 3319 CD Ouhoek Mt Moddergat
7271 maculata 3319 CB N Kwaggaskloof Dam
7273 herbacea 3319 CD Draaivlei Kop
7280 mirabilis 3419 BD N Mierkraal Napier
7283 mirabiis var 3420 AC W Adoonskop
7285 mirabiis var 3420 AC N Brakkloof
7287 mirabiis var 3420 AC SW Adoonskop roadside
7288 mirabilis var 3419 BC N Diepkloof, Oudebakh.
7295 mirabilis var 3419 BC N Diepkloof, Oudebakh.
7299 rossouwii 3420 AC NW Soutkloof
7325 maraisii 3320 CC Laastewater
7327 maraisii 3320 CC 4km E Bonnievale
7334 maraisii 3420 CA 4km NE Bredasdorp
7337 heidelbergensis var scabra 3420 BC SE Brakfontein
7356 mirabilis var 3420 BC NE Buffelsfontein
7376 decipiens Scholzkloof PAlb.
7376 marumiana var viridis Scholzkloof PAlb
7377 viscosa Scholzkloof PAlb
7382 nortieri ‘devriesii’ N Prince Albert
7383 viscosa Syferfontein PAlb.
7386 nigra Toekomst BWest
7417 floribunda/choracantha 3422 BD Melkhoutfontein
7419 venosa subsp granulate 3421 BA Die Eiland, Gouritz
7420 chloracantha 3421 BA Die Eiland, Gouritz
7425 chloracantha 3422 AA Wolwedans Dam
7453 marginataXminima NE Bredasdorp
7485 herbacea 3319 DC Droogerivierberg
7486 cooperi var pilifera Vyeboomfontein, Kaboega
7487 floribunda 3420 BC Byneskop, Potberg
7488 minima 3420 BC Byneskop, Potberg
7482 floribunda 3420 BC SE Klipfontein, Potberg
7494 floribunda 3420 BC +SE Klipfontein
7495 floribunda 3420 BC Matjieskloof, Potberg
7496 maraisii cf atrofusca 3420 BC Matjieskloof, Potberg
7497 floribunda 3420 BC SE Kleinberg
7499 floribunda/heidelbergensis 3420 BC Juliesfontein, Potberg
7500 mirabilis/mutica 3420 AD Die Kop, Wydgelee
7502 mirabilis 3420 AD W Die Kop
7503 mirabilis 3420 AD Mid Die Kop
7504 mirabilis 3420 AD N Die Kop
7507 pygmaea ‘fusca’ 3220 AD Paulsfontein Albertinia
7508 pygmaea ‘dekenahii’ 3421 BA Draaihoek
7509 floribunda 3421 BA Draaihoek
7510 maraisii NE Bredasdorp
7511 variegata 3421 BA Swartklip, Albertinia
7512 turgida 3421 BA Draaihoek
7513 maraisii Klippoort,N Bromberg
7514 mirabilis ‘pilosa’ 3420 AD Stoffelsrivier, S Malgas
7515 variegata 3420 AD Stoffelsrivier
7516 maraisii/floribunda 3420 AD W Kleinberg, Malgas
7518 variegata 3420 AD S Kleinberg
7519 minima 3420 AD S Kleinberg
7520 mirabilis ‘pilosa’ 3420 AD NW7514
7522 nortieri Arizona, Vanrhynsdorp
7523 nortieri Nawelskloof, Gifberg
7524 nortieri Spotzkop, Vlanwilliam
7525 nortieri 17km S Clanwilliam
7526 maculata Western Die nekkies
7577 arachnoidea Nuweplaas, Nieuwoudtville.
7578 nortieri Nuweplaas, Nieuwoudtville
7579 nortieri Trawal Bridge
7580 nortieri Bakkamerfontein, Cedarberg
7586 transiens/cooperi Kliprivier, Uniondale
7593 aristata De Plaat t.o. Kaboega
7594 nortieri ‘albispina’ Koup
7595 nortieri N Dwarsrivier, Cedarberg
7597 nortieri Kunye, Citrusdal
7599 nortieri S Kunye
7604 minima NW Bredasdorp
7605 mirabilis NW Bredasdorp
7608 mirabilis ‘pilosa’ Melkhoutrivier, Malgas.
7609 mirabilis ‘pilosa’ Melkhoutrivier, Malgas
7612 mirabilis Diamant, Swellendam
7615 mirabilis Aalwee, Malgas
7622 maraisii Bergendal, E Ashton
7623 maraisii Sarahrivier, E Ashton
7626 mirabilis Diamant E 7612
7633 mirabilis Luiperdsberg, Swellendam
7634 cooperi cf cymbiformis Glen Avon falls S East.
7635 sordida Zwartkop, Kaboega
7636 cooperi cf cymbiformis N Zwartkop, Kaboega
7640 venosa subsp granulata Oskopvlakte, E Laingsburg
7641 viscosa Blokhuis, E Laingsburg
7642 wittebergensis Ezelfontein, Laingsburg
7644 arachnoidea Bakoven
7646 arachnoidea Bizaansgat
7658 arachnoidea SW Perdekraal
7659 venosa subspgranulata Bantamsfontein
7660 arachnoidea Bantamsfontein
7666 arachnoidea Patatsrivier
7670 arachnoidea Keurkloof, Matjedfontein
7671 arachnoidea Bulhouer, Matjesfontein
7672 arachnoidea NW farmhouse do
7673 arachnoidea N Bulhouer.
7676 arachnoidea N Bulhouer/S Dwarsindieweg
7677 arachnoidea S Dwarsindieweg
7678 arachnoidea SW Josefskraal
7679 arachnoidea Lospersberg, Laingsburg
7681 marumiana ‘archeri’ Lospersberg
7686 arachnoidea NE Dwarsindieweg
7688 arachnoidea Klein Tafelberg, N Dwarsindieweg.
7692 nortieri ‘pehlemanniae’ Volstruisfontein, n Matjesfontein
7693 arachnoidea Volstruisfontein
7695 glauca SE Darlington Dam/Kirkwood.
7698 aristata 1km E Buffelsnek, Kaboega
7700 glauca Above Kaboega farmhse
7701 cooperi’puberula’ Above Klipfontein farmhse
7703 aristata 1km N Zwartkop, Kaboega
4. J.D.Venter numbers for MBB accessions:-
│ 86/68 2551 │ 96/55 6542 │ 97/48 6682│
│ 87/51 3670 │ 96/57 6554 │ 97/50 6684│
│ 87/56 2011 │ 96/58 6552 │ 97/51 6685│
│ 87/57 2373 │ 96/59 6555 │ 97/52 6686│
│ 87/58 2356 │ 96/60 6557 │ 97/53 6683│
│ 87/59 2038 │ 96/61 6561 │ 97/58 6691│
│ 87/62 5209 │ 96/62 6559 │ 97/59 6692│
│ 87/65 2347 │ 96/63 6559 │ 97/78 6720│
│ 87/66 2457 │ 96/64 6563 │ 97/79 6714│
│ 87/72 1247 │ 96/65 6563 │ 97/80 6710│
│ 87/73 2579 │ 96/66 6567 │ 97/81 6711│
│ 87/74 3363 │ 96/67 6570 │ 97/82 6712│
│ 87/78 1119 │ 96/68 6572 │ 97/83 6713│
│ 87/81 2070 │ 96/69 6571 │ 97/84 6694│
│ 87/85 2052 │ 96/70 6573 │ 97/85 6702│
│ 87/90 2092 │ 96/71 6580 │ 97/86 6696│
│ 87/104 2556 │ 96/72 6583 │ 97/87 6696│
│ 87/109 5620 │ 96/73 6584 │ 97/88 6698│
│ 87/131 2292 │ 96/74 6584a│ 97/89 6697│
│ 87/133 2261 │ 96/76 6588 │ 97/90 6693│
│ 93/31 2292 │ 96/77 6590 │ 97/91 6703│
│ 94/75 2292 │ 96/78 6591 │ 97/92 6704│
│ 96/6 6505 │ 96/79 6592 │ 97/93 6705│
│ 96/7 6506 │ 97/22 6624 │ 97/94 6706│
│ 96/8 6509 │ 97/24 6632 │ 97/95 6723│
│ 96/9 6510 │ 97/25 6630 │ 97/129 6741│
│ 96/15 6516 │ 97/26 6641 │ 97/130 6740│
│ 96/16 6536 │ 97/30 6668 │ 97/144 6757│
│ 96/17 6513 │ 97/31 6661 │ 97/151 6758│
│ 96/18 6533 │ 97/32 6660 │ 97/152 6761│
│ 96/19 6521 │ 97/33 6655 │ 97/153 6760│
│ 96/20 6518 │ 97/33 6656 │ 97/154 6764│
│ 96/21 6528 │ 97/33 6657 │ 97/155 6774│
│ 96/22 6527 │ 97/34 6658 │ 97/156 6775│
│ 96/23 6519 │ 97/35 6663 │ 97/157 6772│
│ 96/24 6520 │ 97/36 6649 │ 97/158 6773│
│ 96/25 6534 │ 97/37 6659 │ 97/159 6768│
│ 96/27 6512 │ 97/38 6670 │ 97/160 6769│
│ 96/49 6539 │ 97/39 6667 │ 97/161 6770│
│ 96/50 6537 │ 97/42 6648 │ 97/162 6771│
│ 96/51 6541 │ 97/43 6646 │ 97/163 6776│
│ 96/52 6546 │ 97/45 6673 │ 97/164 6778│
│ 96/53 6544 │ 97/46 6680 │ │
│ 96/54 6545 │ 97/47 6681 │ │
4. JDV numbers against MBB nos.
│ 86/68 2551 │ 96/55 6542 │ 97/48 6682│
│ 87/51 3670 │ 96/57 6554 │ 97/50 6684│
│ 87/56 2011 │ 96/58 6552 │ 97/51 6685│
│ 87/57 2373 │ 96/59 6555 │ 97/52 6686│
│ 87/58 2356 │ 96/60 6557 │ 97/53 6683│
│ 87/59 2038 │ 96/61 6561 │ 97/58 6691│
│ 87/62 5209 │ 96/62 6559 │ 97/59 6692│
│ 87/65 2347 │ 96/63 6559 │ 97/78 6720│
│ 87/66 2457 │ 96/64 6563 │ 97/79 6714│
│ 87/72 1247 │ 96/65 6563 │ 97/80 6710│
│ 87/73 2579 │ 96/66 6567 │ 97/81 6711│
│ 87/74 3363 │ 96/67 6570 │ 97/82 6712│
│ 87/78 1119 │ 96/68 6572 │ 97/83 6713│
│ 87/81 2070 │ 96/69 6571 │ 97/84 6694│
│ 87/85 2052 │ 96/70 6573 │ 97/85 6702│
│ 87/90 2092 │ 96/71 6580 │ 97/86 6696│
│ 87/104 2556 │ 96/72 6583 │ 97/87 6696│
│ 87/109 5620 │ 96/73 6584 │ 97/88 6698│
│ 87/131 2292 │ 96/74 6584a │ 97/89 6697│
│ 87/133 2261 │ 96/76 6588 │ 97/90 6693│
│ 93/31 2292 │ 96/77 6590 │ 97/91 6703│
│ 94/75 2292 │ 96/78 6591 │ 97/92 6704│
│ 96/6 6505 │ 96/79 6592 │ 97/93 6705│
│ 96/7 6506 │ 97/22 6624 │ 97/94 6706│
│ 96/8 6509 │ 97/24 6632 │ 97/95 6723│
│ 96/9 6510 │ 97/25 6630 │ 97/129 6741│
│ 96/15 6516 │ 97/26 6641 │ 97/130 6740│
│ 96/16 6536 │ 97/30 6668 │ 97/144 6757│
│ 96/17 6513 │ 97/31 6661 │ 97/151 6758│
│ 96/18 6533 │ 97/32 6660 │ 97/152 6761│
│ 96/19 6521 │ 97/33 6655 │ 97/153 6760│
│ 96/20 6518 │ 97/33 6656 │ 97/154 6764│
│ 96/21 6528 │ 97/33 6657 │ 97/155 6774│
│ 96/22 6527 │ 97/34 6658 │ 97/156 6775│
│ 96/23 6519 │ 97/35 6663 │ 97/157 6772│
│ 96/24 6520 │ 97/36 6649 │ 97/158 6773│
│ 96/25 6534 │ 97/37 6659 │ 97/159 6768│
│ 96/27 6512 │ 97/38 6670 │ 97/160 6769│
│ 96/49 6539 │ 97/39 6667 │ 97/161 6770│
│ 96/50 6537 │ 97/42 6648 │ 97/162 6771│
│ 96/51 6541 │ 97/43 6646 │ 97/163 6776│
│ 96/52 6546 │ 97/45 6673 │ 97/164 6778│
│ 96/53 6544 │ 97/46 6680 │ │
│ 96/54 6545 │ 97/47 6681 │ │