- Haworthia Update+ – Table of Contents
- Van Reenens Crest
- Aloe pumila, Haworthia pumila; what or who is confused?
- Haworthia mirabilis magnifica, MBB6651, S Riversdale
- The Haworthia pollinator
- Haworthia limifolia – a conundrum or a lesson?
- Very brief note re Haworthia nortieri flowers
- Haworthia marxii and H. truteriorum in relation to rational classification.
- The reality of Drosanthemum micans L.
- New finds in Haworthia
- What do collectors need?
- Righting some misconceptions
- Comments on H. marxii
- Haworthia maculata ↔ Haworthia pubescens, MBB8002 Cilmor
- Addendum Haworthia pubescens MBB8011, SW Sandberg
- Genetic history – the place in Haworthia classification.
- Haworthia mutica (groenewaldii) and its twisted leaves.
- Comprehension and significance
- Habitats
- MBB6694 Kanetvlei, Hex River Valley as a variant of Haworthia nortieri.
- Haworthia minima and pumila flowers
- MBB7989 Haworthia pumila, Lemoenpoort
- Leaf arrangement in Aloe striatula
- Just what do we do with names for Haworthia?
- Nomenclator (2013)
- The absurdity of taxonomy and nomenclature?
- Variable chloracantha
- Leaf sequence in Haworthia emelyae ‘comptoniana’ over a long period
- The science (of plant names)
- What do you think this is?
- Kaboega
- Dunning-Kruger Effect
- Flowering Time
- Morphology
- Fusca
- Cooper Siding: Darkness of Ignorance!
- Species, what species?
- Taxonomy and Fieldwork
- Zuurberg cooperoid
- Maculata
- Clump
- Little physical substance – H. marumiana MBB7681 Lospersberg
- A random note about Haworthia chlorocantha
- Tulista
- Accession
- Cymbiformis Cooperi
- Gulag
- Tulista pumila
- Flowering Time Redux
- Personal Names
- DNA Sequencing
- Mirabilis MBB7513
- Mutica reminiscences
- Closing Thoughts on Haworthia
- Ethical collecting – a conversation
Category Archives: Update +
Van Reenens Crest
Photos from a field trip to the farm Van Reenes Crest
(half way between Swellendam & Heidelberg along the N2).
See an earlier article in Haworthia Updates Vol. 7 Chapter 6.
Trevenen & Hesphia Barry
Honey Guide Cabin
P.O. Box 8, Buffeljagsrivier. 6742
084 516 3596

Aloe pumila, Haworthia pumila; what or who is confused?
With some astonishment I learned recently of an opinion that the name pumila as used in Haworthia is confused and therefore should be discarded. There is no doubt that it is difficult to unravel the literature and the usages of the name, but I think there are a number of important questions which should first be answered. Is the name confused or is it us and others, as individuals, who are confused? It is worth asking such questions because names are words in the process of communication and mutual understanding around which all knowledge and its sharing revolves.
If the facts of the matter are properly examined there is a clear path of events. It may be very complex and take many words to explain, but it is there. If a reason has to be sought for confusion I have no doubt that it can be tracked to the door of the International Code of Botanical Nomenclature. My reasons for saying so should become clear.
Paul Forster, who is himself a professional and highly qualified taxonomist, commented on my treatment of the name H. pumila as follows “We do not live in a vacuum.” Here he suggested that I had failed to consult authority and that there was professional opinion available that would have resolved my confusion.
If one thinks about Paul Foster’s statement it is quite evident that:
1. he does not consider himself able to resolve the problem (this is a valid point because to do so does require knowledge of the elements involved, and also requires insights into the intricate mechanisms of the Code which are beyond most).
2. he simply believes that there is someone in the hierarchy who is competent and able to do so.
3. I had not thought there was.
The latter point is simply not true as I have consulted almost every single botanist and non-botanist (yes, many non-botanists are free to dabble in plant taxonomy because of the non-scientific nature of the activity) available to me. A list of names and also authors and published references would fill a few pages.
Turning to the name “pumila” itself, it can be shown very clearly (botanical terminology to mean something quite the opposite, but which I use here in the true sense of understandable) that Linnaeus used the name for four “varieties” of small Aloe. Confusion certainly followed about which of his names should be used, but there should be no doubt about which four these were.
The problem arises out of the Code and how, relating to names for those four “species”, it is now interpreted and by whom. It becomes a juristic problem requiring a great deal of fancy intellectual footwork, complicated by an approach to the code which largely ignores the basic intention to bring stability and uniformity to plant names. It also involves use of words like “validity”, “legitimacy”, “intention”, “relevance”, “strict interpretation” and “according to article…” with gay abandon.
Without going into the very extensive historical detail which includes the fact that the names margaritifera and pumila were used for the same single thing, it is almost sufficient to say that Col. C.L. Scott took the plunge. Defying the predictable response, he took the assistance of Dr. L.E. Codd, who was then and still remains a highly respected taxonomists. Together they concluded that because of what and despite what, anyone had done with those two names, the name “pumila” was valid and correct for the species represented by the illustration t10 of Commelin 1701.
They argued that despite the fact that the name had already been used in Haworthia for another species, this did not preclude its correct use for the one represented by the Commelin illustration. The fact that confusion may have arisen and continues to this day, is to my mind correctly laid at the door of Dr. W.T. Stearn, paragon and patron saint of plant taxonomy. He wrote a paper in 1938 in which he annotated the names of Salm-Dyck and reconciled them with Berger’s revision of Aloe in 1908. What he did was also to typify the name Haworthia herbacea (Miller) Stearn. In my opinion, had he known anything about the actual species involved, he would have recognised the problem arising from the inclusion of “pumila” in the history of that name and resolved it accordingly. He did not.
After Scott (and Dr. Codd) had reached a decision in 1978, I still remained in some doubt using the name “pumila” until forced into a decision when I undertook to write a revision of the genus myself. I had been asked to write a synopsis of the species of Haworthia occurring in the Cape Floral region. This request was by the taxonomists Dr. J. Manning and Dr. P. Goldblatt and I in turn asked them (as had become my routine practise with taxonomists) to clarify the use of the name “pumila”. They contacted Dr. Fred Barrie who I presume is a figure in the hierarchy alluded to by Paul Foster, and the following was his reply. “According to the Linnaean Typification Project database, Aloe pumila var. pumila was lectotypified by Wijnands (Taxon 34:310, 1985) on Commelin, Hort.Med.Amstel.2:t.10, 1701. Linnaeus cited this figure under A. pumila var. margaritifera in Species Plantarum (p322) as “Comm.hort.2,p.19,t.10.” Consequently, var. margaritifera and the autonym are synonymous. Var. pumila, as the autonym, has priority.”
There is more to the reply, but it does not address the problem of the different usages of the name “pumila” by various authors until its inclusion in Haworthia by Duval for a species based on a different type.
There is no confusion in this. It is simply a question of how the Code directs that the name should be treated. I was confused over the issue. I did wade through the detail of synonymy with Mr. Larry Leach and subsequently with Dr. Peter Bruyns. It was evident from this arduous process that, was “pumila” not available for the species in question, the name “maxima” should be used. There is absolutely no reason for confusion about which species I am referring to, nor about the names available or the way in which they have been used since Scott 1978. But I decided to treat the name “pumila” according to Dr. Barrie’s response. This suggested to me that Dr. Codd and Co.l Scott were correct and that “pumila” as used by Aiton and Duval was incorrect and did not preclude the use of the name as typified by Wijnands. The essence is that Linnaeus used the name “pumila” as a prime name in Haworthia and it should sensibly remain there.
What is confusing and what should confuse everyone, is that there are persons who feel the need to contest the issue. The need only arises from personal feelings and the fact that the Code has generated this vast arena for endless vain debate. Where a name has so convincingly and obviously been used in the literature of the time, it obviously meets the need. Changing it, or attempting to change it, generates confusion. We do not have to feed on it. ♦
Haworthia mirabilis magnifica, MBB6651, S Riversdale
I think we have to find a way to deal with this issue of names. Let us try H. magnifica. It originated in a population from the Frehse Reserve SE Riversdale and it is truly difficult to circumscribe all those individual variants. The name has also been attached to a number of other plants and populations from various places. The name magnifica is not clearly assignable beyond individuals that can be said to resemble the type (an illustration) and there are individual plants in the Frehse population that do not accord with either picture or description. There are individual plants and populations going all the way to near Caledon that confound the name still further. In my opinion the Frehse reserve plants belong to a single system that I consider to be H. mirabilis (and I am not so sure that it is not bigger still). Do we just drop the name ‘magnifica’ and use locality? So is it better to say H. mirabilis magnifica (Frehse Reserve) and H. mirabilis magnifica (3km S Riversdale) or H. mirabilis magnifica (Windsor) for the variants that occur at each of those places? Also H. mirabilis jakubii (Goukou) that I think is a connection between H. mirabilis magnifica and H. mirabilis paradoxa (Vermaaklikheid and on to Infanta). We could use this system and also the Breuer and Hayashi names attached to other mirabilis populations in the Riversdale area. The disadvantage of place names is that they convey nothing to people unfamiliar with local geography and to them there may be no difference. On the other hand that the formal Latin names may be restricted by the accompanying description and illustration, and not convey the variations that occur in the various populations.


















































♦
The Haworthia pollinator
While I have seen and recorded a Solitary Bee species pollinating the flowers of Haworthia, I have never succeeded in photographing this pollinator. Slightly larger than the ordinary Honey Bee, it is a very rapid and busy flyer and does not spend time at any one flower. Here in Cape Town it is relatively rare and there has been an odd season where I have not seen it at all. It makes a nest consisting of a short tunnel dug into the ground where it makes a series of nectar and pollen filled cells in which the eggs are laid. At Worcester these insects were very common and on one occasion I came across them nesting in large numbers in a small patch of bare gravelly clay. It is remarkable how they were obviously able to recognize and home in on their own small tunnels. It does come into relatively insect proof enclosures and can make isolation of plants for pollination difficult. It is unlikely that flying distance has been measured and it seems very unlikely that it will match the observed maximum of the Honey Bee at 13km (8miles). However, I do not know the length of the life cycle and the relation of the feeding/foraging activity to its nesting behavior. It may be possible that feeding but non-nesting bees disperse over greater distances.
The chance to take the two photographs presented here came quite by chance. I was in my plant house that is partly accessible to insects, and saw the bee on a Haworthia flower stalk. Its jaws were clamped on the peduncle and it was totally stationary. I took a few pictures and then attempted one from another angle. In doing so I had to dislodge a second peduncle from nearly under the bee. The slight difficulty in doing so did not register properly. I took a picture and then suddenly the bee came to life and flew off to resume foraging in the house. But it soon settled on another inflorescence (on a plant at another table) again on the peduncle with its jaws clamped on the talk as before. I removed some pollen coated threads hanging from its back legs and left. While downloading the pictures I saw that I had photographed a spider on the peduncle under the bee in my second angle. So went back to see. The bee was still stationary on the flower and then I saw what I thought was the same spider crawling over the bee. Somehow I disturbed the bee again and it flew off up into the shade cloth in seemingly healthy fashion. The next day I thought I would check the original peduncle to see if there was any web. Much to my surprise I saw the original spider and no web. I have seen these small yellowish spiders on flowers before and also noted small amounts of web. Very occasionally there have been dead flies sitting attached to the flower stalks but not with any noticeable web.
So it is still a bit of a mystery if this small spider, or spider pair, could actually capture and immobilize such a bigger potential prey. Certainly the bee I observed had encountered web and may have grasped the stalk to free its hind legs to work the web off. I did not observe anything more than minimal movement to do so.
Regarding pollination. I was not doing any controlled pollination during which I would have been checking for the presence and exclusion of the bee. But what I did observe was seed set on H. limifolia clones that I could not achieve although in desperation I have transferred pollen between different collections. The bee had an advantage in also bringing pollen from unrelated species. This was on H. limifolia ‘gigantea’ and on H. limifolia ‘glaucophylla’. While I have set a few capsules on the former, I have achieved none on the latter. What is interesting is that no seed was set on field collected clones of H. limifolia presumably ‘keithii’ from Isiteki.
During feeding/foraging, the bee holds onto the lower flower limbs and very briefly inserts its ‘tongue’ into the flower and buzzes away. I could not see any deliberate hovering and pollen transfer to the back legs as does the Honey Bee. But pollen is collected and carried in approximately the same way. When I hand-pollinate I adopt the simple approach of approximating the insertion of hair (bees ‘tongue’) into one flower to obtain pollen and then into another to deposit it. Better results are claimed for physically exposing the stigma and transferring pollen using a brush.
A recent trip to photograph flowers resulted in us finding H. floribunda southeast of the Bontebok Park at Swellendam. I have reported elsewhere that I had seen this species within the park but repeated visits had turned up nothing but similar looking H. mirabilis. On this occasion we were outside the park where we have recorded H. mutica, H. marginata, H. minima, and also similar small forms of H. mirabilis as occur inside the park. We explored a small area we had not covered before and were delighted to find H. floribunda, now easy to see because they were in flower. However, we proceeded to the H. mirabilis locality and on the way again found H. floribunda that we had missed on the previous visit. We also found more H. mirabilis about 60m further along and still about 100m away from the original H. mirabilis locality. These four species were along a stretch of about 5-600m and not occupying shared habitat. The vegetation was fynbos on shallow alluvium, quite stony and well-drained.
It was while photographing the flowers of H. floribunda that a furry fly appeared and ignoring us and the camera, attended to the flowers. While I was trying to get a picture of this fairly fast moving fly, the Anthophorid bee pollinator also appeared but flyng too fast and haphazardly to be pictured. We had seen the same fly on H. mutica at the Buffeljags habitat. The species is Australoechis hirtus known to be a pollinator and visitor to many flower kinds. The flies are nectar feeders and parasitize other insects in their reproductive process.
While we do not know what the pollinating effectiveness of these insects over distance is, it is quite obvious that in this situation there must be substantial transfer of pollen across all the species given the short distances involved. Three of the species were in flower. But H. marginata, curiously, at this locality flowers nearly 5 months earlier than at other known places. Despite that, there are hybrids with H. minima. There did not seem to be hybrids among the H. floribunda plants, but we did think that some of the plants of H. mirabilis could have been hybrid. ♦




Haworthia limifolia – a conundrum or a lesson?
I was contemplating the problem I see in the way we approach classification, contending that there is no resolution to the endless addition of new names and arguments about their validity and usefulness unless we reach some agreement on method and purpose. The confusion about names, descriptions and identifications that had arisen in 1947 came about for four reasons. Small samples and inadequate exploration, too many unqualified experts, lack of insight into what species actually may be, poor methodology.
There is no excuse for this situation to continue. Sampling is at a very high level and because the other three elements have not been removed, confusion is as great as before. Many names may have been lost from sight, but there is no lack of new ones to replace them. Experts are a problem and their qualifications even more so. In my own experience I have communicated with many highly qualified botanists who have contributed absolutely nothing to the resolution of problems as basic as typification of names, or definition of the word ‘species’. So what then contributes to qualification? This is surely an unbridgeable problem when amateurs have dabbled so freely in plant classification with apparent success. But their success has come from the failure of qualified botanists to put something more substantial in place than a nomenclatural code for binomials and nothing to indicate what those should mean, or who is competent to generate or change them.
Neither of those problems can be solved by me. My contribution has been to see as much as I can of what others have seen, and then to myself explore in both the arena of the subject matter and the periphery of biology that touches it. What I have done is to try and relate what I have read and been taught to what I have observed and experienced. In this way “species” have become a reality as systems of populations and individuals that are spread in space and change with time. Whether evolution is a fact or not I cannot say, but change certainly is. Classification since Linnaeus, has always been associated with this change but not necessarily by everyone. But in the time of Linnaeus, the sampling level was very low and anything new could be, or was, interpreted as a new species and diagnostics were put in place to aid identification. Hence even the Latin binomial was a diagnostic. As the sampling level has been raised so has the system come under increasing stress and it is a fallacy to think that only Haworthia is problematic. However, this is the genus under discussion here and we have to admit that the diagnostics and the methodology do not work.
H. limifolia can be used as an example to illustrate the situation.
It is to all intents isolated from the rest of the genus. We do have H. koelmanniorum sitting to the far north-west and then H. tessellata further to the south-west. Otherwise the rest of the genus is in the Cape. The issue of subgenus need not be seen as a consideration here even though the differences there are as great as any elsewhere in the family.
H. limifolia has a very wide distribution from Komatipoort in the north, westwards and southwards to Vryheid, eastwards and southwards to Gollel and then Stanger in KwazuluNatal. The whole of Swaziland and a large part of northern KwazuluNatal may harbor this species. We need to even ask … “Is it a species?”
The fact is that there are very different elements involved in respect of individual appearance and virtually all aspects of that individuality. The possibility is that whole populations may be derived from vegetatively propagating individual clones. Some plants are solitary and propagate with difficulty. Others proliferate as vegetative offsets or by stolons. The leaf shape and surfaces are highly variable. The surfaces may be flat or channeled; absolutely smooth, to having raised wave-like wrinkles, to tuberculate with small individual tubercles, or the tubercles may be fused in groups and ridges. These may be normal leaf-coloured or they may be white. Flowers are extremely variable and so are the seeds and the capsules. Flowering time seems to be opportunistic and in cultivation the plants may flower at any time, usually synchronizing during the summer months.
Do we really have any alternative but to see this as one single very large operating systems, despite all the real problems of isolation by distance and hence the associated problem of gene transfer between individuals and populations? Is it sensible or even practical to insist or suggest that there must be Latin binomials that describe (in the early Linnaean sense) the situation or, more importantly, that there are diagnostic characters by which such single taxa (groups, species, varieties, forms etc) can be identified?
How can the situation we observe in this relatively isolated system and (species?) be extrapolated to the Cape where all three subgenera and the bulk of the species occur?
Recently two authors have proclaimed the value of floral morphology in resolving this ongoing disputation about the species. What their actual view of what species are has not been spelled out but it appears to be a belief that simple structural (morphological) differences are what define them. Behind this must lie the unconscious acceptance of the original definition of species that they were systems of interbreeding or potentially interbreeding organisms. Thus flower structure is assumed to be linked to the breeding system and therefore very important in classification. This view of species still seems to be the power behind classification although it has long been recognized that in plants inter-fertility may extend across generic difference.
In Haworthia the flowers, even across the sub-genera, are fairly similar and especially in regard to size. Within the sub-genera, the similarities between what are considered to be species, is remarkable. There are even exact similarities that can be found in what are truly geographically and vegetative different populations. Flowering time seems to suggest some kind of additional factor but even here it is evident that breeding can occur between populations with flowering times 6 months apart. It has to be considered that flowering time differences like this might even be a characteristic of a species. This has in fact been shown for H. marginata and H. minima that surely can otherwise seen to be different systems.
The pollinating agents are insects and it seems as if flower size may be linked to that fact as it is so consistent across the genera and whatever are thought to be the species. Here I just present a number of pictures of the profiles and faces of flowers from 5 populations H. limifolia of which some are from different clones in those populations. A difficulty in viewing flowers is that they change with age, and place on the peduncle. They only last about three days and it is thus difficult to compare flowers of exactly the same age and condition. Of course these are poor pictures but no essential and detail is lost here by poor focus. But it is quite obvious that there is bigger difference between the two flowers (profiles) of the plants from Ithala than there is between them and other populations. Consider too that it can be very difficult to distinguish any of these flowers from those of other species in the subgenus Hexangulares. This essay is not submitted as conclusive evidence and argument and I will enlarge on this in a following essay. ♦

















Very brief note re Haworthia nortieri flowers
Some criticism about my supposedly having ignored the flowers in Haworthia comes at a very inopportune time. I set aside flowers for the reasons very obvious from the historical record but also because of the considerable problems of similarity in the appearance of the flowers in apparently quite different species. My priority was a geographic overview and a rational basis on which discussion and decision making could be based. It I just grossly unfortunate that other writers and critics seem to be wedded to a classification paradigm locked into the approach that prevailed 70 years and more ago. This in the total absence of a species definition other than the vague acceptance of a zoological one based on interbreeding capabilities. This ignoring the ease of hybridization among Haworthia variants in general.
While I have written an account of flower appearances in a small selection of populations, I also came across these few images I have of flowers in what I regard as the species H. nortieri. I have also added images of a single flower of H. maculata from a population high in the mountains at Worcester that could be seen as a southern extension of the H. nortieri set of populations. Note must be taken of my early contention that H. nortieri and H. globosiflora were the same species, based on my observation of the intermediate appearance of the flowers of a Vanrhyns Pass population. The H. maculata bud is typical of the species in the Southern Cape, whereas H. nortieri has rounded bud-tips.
The flower of the Trawal plant are dramatically different from that of, say, Sneeuberg. It is very understandable that differences like this lie at the base of all the argumentation and confusion that so despoils the naming and identification of Haworthia. A classification has been needed against which to explore and examine these differences. It seems to me totally unnecessary to try and construct another hierarchy of solely Latin names while so little is still unknown. ♦















♦
Haworthia marxii and H. truteriorum in relation to rational classification.
Explanatory note: In a rationalized list of names I wrote and had published in Haworthia Update Vol 7. I made two decisions. One of these was in respect of H. marxii and the other in respect of H. truteriorum. In the case of H. marxi I included it under H. emelyae. In the case of H. truteriorum, I placed this under H. bayeri. In neither case did I have good evidence for doing so, much other than my conviction that a classification is intended to reflect origins and relationships. In formalizing names one perforce is pressured into making decisions that you are not informed enough to make. My main defense is that the authors of the two species were not adequately informed either. I think their account of H. mutica (their H. groenewaldii) at Buffeljags demonstrates this. H. marxii presents particular problems that I simply do not have any substantial data that I can process. In the case of H. truteriorum I did have some to which I can now add. This suggests as Breuer and Marx indirectly indicate, that H. truteriorum relates to my concept of H. mirabilis. I concede that I may be quite wrong in attributing it to H. bayeri. I find it very difficult to see the decision to describe it as a distinct species as a logical scientific action. This article does not extol any imagined virtues or skills of mine. It is only intended to further project my opinion that we urgently need to work towards a classification that does satisfy scientific principle and not novelty or commercial ends. It is also a counter to some very negative opinion aimed in my direction.
At a level above classification lies respect for people and their feelings. Therefore this writing should not be seen as anything but a commentary on the classification process and not how this can also denigrate people as much as honor them. H. marxii is described by Sean Geldenhuys in ALOE 44.1:5, 2007 from apparently 2 populations in a confined area in the north and east of the Little Karoo. Placing it with H. emelyae simply reflects doubts that need to be expressed about how this oddity has come about, and respect lost for reasons not necessary to explain. H. truteriorum is described by Ingo Breuer and Gerhard Marx in ALOE 48.3:54, 2012 and refers to a single population of plants southeast of Oudtshoorn. That this latter population is singular and extremely interesting goes without question except for how it is explained. Despite much correspondence with both authors and after reading their published works, I am not aware that either has a concept of what a species is any different than what may have been held by either K. Von Poellnitz or G.G. Smith. Ingo Breuer particularly has published what he refers to as a species concept of Haworthia. This is nothing more than a long list of Latin binomials and we have to assume that this is then also a list of real species whatever they might be. Gerhard Marx maintains that my attempt at a species definition is so broad as to be meaningless. He has not supplied an alternative definition and I am left with the impression that he makes the same assumptions that “character” differences equate species as does the ordinary uninformed mortal.
H. truteriorum is described in a popular journal (Aloe) that has been approached by at least one botanist not to publish new species as there is no official review process. However, the response was that the journal does have its own in-house authorities that cover the possibilities of scientific lapse. I note that the article is specifically foot-noted to indicate an editorial review and I perforce do not see this as a check on the scientific content. There are some lapses that I will deal with but recognizing that these might not be the same ones that a properly qualified botanist (a minimum of a recognized 4-year degree course), nor those that an experienced and knowledgeable botanical taxonomist may have corrected. More important though is the population itself and where it fits into the overall Haworthia picture.
The most substantial gain that we can make is to arrive at a species definition. It is evident to me that species are complex systems in which there are variations that have arisen from to earth differences and must continue to exist to facilitate response. If there is evolution and thus adaptation and selection, then there has to be something to work with. The phylogenetic idiom was “specialization is precursor to extinction”. Geological and habitat diversity result in plant diversity. This diversity is necessary for survival as the habitat changes. Therefore a species will have a geographic distribution across which individuals and populations will vary and be different from one another. Geology and geomorphology will be strong factors influencing plants like Haworthia that are associated with skeletal soils and rocky habitats. This means that we have to look for associations in respect of distributions and the driving forces that affect even vegetation. Thus in the case of H. marxii (known from 2 populations in a small area south of Laingsburg) the vegetative appearance of the plants cannot be seen in any other species geographically closer than H. emelyae, which is quite a long distance away southwards. The presence of H. pumila in the same area as H. marxii suggests that the Haworthia presence there could extend from the Worcester/Robertson Karoo. Hence H. mirabilis, also present in the Montagu area, cannot be ruled out in seeking a relation to H. marxii. There is much more to this issue of H. mirabilis in respect of its variability and its distribution into the Little Karoo that impacts directly on this suggestion. It also impacts on the real identity of H. truteriorum.
Where I see a real problem with H. marxii is in floral morphology. I gather that the flower very much resembles that of H. marumiana dimorpha. In the same way that the flower of H. pulchella globifera is identical to that of H. cymbiformis incurvula at Plutos Vale, there is a massive problem in drawing conclusions from flowers as a character for the level at which all role players are trying to identify species. But so-much for H. marxii and I do not seriously question it.
The case of H. truteriorum is more manageable. I have been speculating for a long time that H. mirabilis and H. emelaye may in fact be the same species. This may be the proper level at which we should be recognizing species as systems. I extend the argument even to say that it is not inconceivable that H retusa and H. mirabilis are one species. This means that I have to bury my long-held objection to the view that “Haworthia is a genus in a state of active evolution”. My objection being that this is an obvious aspect and that in any case all species are faced with the inevitable need for change and adaptation. But this does not weaken my view that species are chaotic fractal systems that vary around a point of attraction according to the stability of their genetic bases and mutating rate. How strong I am on the technicalities of DNA and evolutionary theory may be problematic, but there is no evidence that other authors even contemplate the issues.
Gerhard Marx is a remarkable observer and I have huge respect for his many skills. Breuer is a really competent compiler too. I do not question what they say about the characters of the plants and their observations. What I question is their knowledge and insight into broader botany and the distribution and variation in Haworthia however much more they know than the above average collector.
My prime objection is that the description involves a single localized population. This in itself creates huge doubts in my mind because on this basis, Breuer’s several hundred species is conservative. I will only dwell on three other points. The one is their very trite “Never before have retusoid type Haworthias been found growing in shale in the Little Karoo”. The second point is the habitat description in relation to geology. The third is the flower and flowering time. The fourth is about the illustrations and the art work.
Haworthia mirabilis was recorded in shale at Barrydale by Smith prior to 1947. It was recorded at two places in shale or shale derived soils prior to 1999 and I can add that I have seen it at two new locations in shale in the Montagu area since. The second point is the description where it is referred to H. bayeri and H. emelyae occurring in quartzite and quartzite conglomerates. As in the description of H. groenewaldii, this is simply a very crude and inaccurate account of a very important issue. I am no geologist but I do know what quartz is and that it occurs in both shale and sandstone formations. Furthermore, I do know that the Oudtshoorn area has an incredible geology with ancient and recent geological formations adjoining as a consequence of faulting and folding. Quartz is Silicon Oxide and is apparently soluble in water at high temperature and pressure formation so that it can accumulate in fissures and bands in parent rock. In both sandstone and shale the quartz varies in purity, and the crystals in size. South of the Langeberg the shales are covered in an extensive layer of tertiary gravels that are far less extensive north of the mountains. But the main point is that the vast quartz patches of the Little Karoo are actually associated with quartz existent in the Bokkeveld shale. The “species” need to be properly looked at in their relation to that complex geology that exists there. Marx and Breuer mention the differences north and south of the Outeniqua Mountains. But the Outeniqua Mountains are just an eastern extension of the Langeberg from the Gouritz River gorge. The geology north of that area is quite different from that west of the Gouritz. This suggests an ignorance of geography added to that displayed for the geology.
Checking my own knowledge and experience of the habitats of the species involved (viz. bayeri and emelyae – with the name picta an anomalous insertion), it is very clear that the statement “quartzite and quartzite conglomerates” is erroneous. There are four geological formations involved and these are the Table Mountain Sandstones, Bokkeveld Shales, and then Enon and Tertiary deposits. In the Heimersriver where the H. bayeri, H. emelyae and “H. truteriorum” are reported, lacks Enon presences. The report of the plants in unfragmented, unweathered upright shale (Bokkeveld) needs to note that the quartz patches in the area are the result of fragmented and weathered shale.
Marx and Breuer perhaps should also take account of H. outeniquensis not a great deal further south in an area that I believe is still unexplored. There is also the mystery of a plant found by Avril Schein in that close area that remains unexplained.
We know from the H. retusa – H. mirabilis interaction that flowering time is no barrier to hybridization and that flowering time may not be indicative of a completed speciation process. Ignoring the fact that we can only guess, on the basis of the scientific paradigm, that we are interpreting and trying to understand an evolutionary process. Breuer has attempted to jump this issue by the recognition of “aggregates” and the two authors use the fob of “the mirabilis/maraisii/magnifica complex. This complex I presume is explained as a list of names only. This I do think emanates from a mis- understanding of a real knowledge of field botany generally and of Haworthia in particular. I say this with great emphasis and conviction because it is something I am still working towards. I have just completed a very thorough look at a the flowers of a very small fragment of populations driven by the expressed opinions of both these authors in diverse places, that flowers are significant with the import that I have ignored them. The fact is that if the flowers are considered then we have a bigger problem than before – not a solution. The flowering time of H. truteriorum that Breuer and Marx cite is very possibly an indicator of behavior rather than a species differentiator. Why they emphasize it is most probably, as J. Manning pointed out, most observers (taxonomists) have a subconscious belief that species are things that do not interbreed and so flowering time is seen as such a great barrier to interbreeding that it MUST be a major species indicator. My observation is that this is not true and that species are inherently highly variable systems with great capacity to respond to environmental drama that may threaten their continued existence. The nature of the problem is very well illustrated in the case of H. retusa and H.mirabilis where I contend that a fully developed view of the genus may require that they be seen as one species. Thus the importance of flower and flowering time is part of the myth of a non-existent species definition and is confounded by the concept Breuer and Marx have of the nature of Haworthia species.
There is a very good description of the flower and its character, but the illustration and the art work is weak. The photograph of the flower is of a single dissected flower to show an internal structure that could be of any species in the subgenus and is hardly helpful. That flower does not look to me like the perhaps longer narrower flower of H. mirabilis (real) which is largely recognizable on the arrangement of the petal tips in the bud-stage. This ‘mirabilis’ character should be apparent in the way the petal tips display and we have only a painting to judge this by. Despite Marx’s craftsmanship I am not sure if his flower picture is a true image. I do have an observation on with his outstanding art. Many years ago, photography was a bit of a handicap when it came to illustration of floral and other plant detail. Historically artists were used to capture detail that could not be described or otherwise illustrated. In the present age this is a bit of a myth and it lives on simply because of the skill that is involved in the production of a good piece of botanical art. Marx’s art is up there with the very best. But good art does not equate to good science? Or does it? Botanical art is perhaps not the same as pure art and the intent of either may be quite different. With pure art one could surely be trying to achieve the same goal as science and there must be some trick to understanding the similarity.
My conclusion is to plead for more rational classification and better attention to those things that matter. The fragmentation of a genus by Latin binomials just because of collector and novelty interests, aggravated by commercial implications however slight, is a disservice to all of us. ♦
The reality of Drosanthemum micans L.
Donald A. Levin (2000) quotes Raven, Berlin, and Breedlove (1971), who wrote…“our system of names appears to achieve a reality which it does not in fact possess”. I find this a curious quote, as Levin was discussing species concepts and we could ask if species themselves have any reality. There is a lack of a universal definition for the word “species” and I find the recurring reference to “concept” as related to the word, very confusing. Why should the species be a concept, subject to individual interpretation? This is of course if they have no reality and we each create our own. How useful is this for science? Donald goes on to generate his own “species concept” in which he proposes…“that each species has a unique way of living in and relating to the environment and has a unique genetic system…”, and he refers to this as the eco-genetic species concept. But still we do not know what he means by “species”. He says that the ecological properties of such species are not uniform within the species and thus not equivalent to the taxonomic properties of species, “which are chosen because they are conservative and stable attributes”. It would be interesting to know just what he means by “taxonomic properties” and I presume he means primarily morphological characters. Certainly there must be taxonomists who utilized or have utilized ecological facets to decide on their species. It must also be recognized that the taxonomist is confined to the material at his disposal for examination and decision making.
My view, to the degree that I can understand Levin’s arguments, is that he has not truly stated the case. The problem concerning the reality of Latin names, is that they have largely been generated by taxonomists who may not have recognized that morphological (“conservative and stable attributes”) properties of a species may vary just as substantially as those of eco-genetic species; and also that these “realms” for each species may be different. From my long experience in taxonomy and the usage of names in communication, it is evident that it is the taxonomic system and taxonomists, which have induced the majority of people who use Latin names in any way, to believe that they do have a reality. The system has been based on the view that Levin’s apparently has, that a taxonomist determines species by characters or character sets which can be quantified and easily (sic!) used. Perhaps also, that the taxonomist has had enough material to make a decision that is universally true. In the absence of a universal definition it is self-evident that taxonomists and persons, who use the names they give and are given, may have quite divergent views on what those names actually mean and what reality they have. This is especially true if sight is lost of the fact that the characters taxonomist use and have used either morphological or ecological, are simply not as “conservative or stable” as believed. Neither may they be adequately circumscribable or quantifiable. The material examined may simply be inadequate to convey the varied characteristics of the species as it occurs throughout its distribution range.
I would like to use an example to illustrate the relationship of names to taxonomic characters and identification. Drosanthemum micans is an old Latin name that was given by Linnaeus for a mesembryanthemum species. The name is apparently based on a Dillenius illustration (fig.1). While the name has been commonly used in the ranks of botanists and horticulturists of my personal acquaintance, there is some doubt that the connection of original illustration and name, to the plants identified as such in recent times may be right. In this case, my use of the name stems from the usage of my predecessor at the Karoo Botanic garden, F.J.Stayner, and he probably obtained the name from identification by Mrs. L. Bolus. I many cases names may come into general use through less authoritative channels. At this point in time the true typification of this name and its correct application is under review, so I will use the name as I know it for a species that to my knowledge occurs both within the Karoo Botanic Garden Reserve, a short distance to the east and also in the developed suburb to the northwest of Worcester, Brandwacht. The name “micans” means “glittering”, and indeed this is the case for this plant (fig.2). The species is characterized by inner and outer rings of bright colour, the calyx bears enlarged bladder cells and the leaves have a grey-blue colour in the summer months. The flowers have outer black staminodes and there is a series of inner smaller petals, often uniformly one-colour, which accentuate the coloured ring effect. There is another species with these black stamindodes viz, D. speciosum, which can be separated from D. micans primarily by the appearance and colour of the leaves. In the former the leaves tend to be slightly more globose and have a greenish-yellow colour. In the latter the leaves are grey-green and tend to be elongated with an uncinate (hooked) end. The black staminodes seem to be unique in the mesembryanthema and who knows if they serve any particular function.
In more recent times, Mrs. Bolus described several other species. One is Drosanthemum bellum that is said to have been collected “near Ceres”, and D. hallii from hills east of Rawsonville. D. bellum was described as pink with black staminodes, and D. hallii as yellow with black staminodes. During a period I which I was interested in these very colourful plants, I came across a population of plants at one spot on the hills “east of Rawsonville” (Die Nekkies, north of the Brandvlei Dam) that demonstrated an array of colour forms. Among these were pink (bellum – fig.3), white, and purple. At the same time I found a population of plants nearby in which all the flowers were bright yellow (hallii – fig.4). More recently I have had occasion to examine this more closely and find many more populations, which indicate the problem that taxonomists have apparently yet to come to grips with if botanists and scientists in other disciplines are to find any reality in among Latin binomials. This is that these characters are not diagnostic for the “species”
On revisiting the “bellum” population I found the same array of colours. There were plants with very pale yellow flowers, pink, pale rose-red, white and white shade with purple (figs.5, 6, 7 & 8). There were no bright yellow flowers despite the fact that such bright yellow flowers characterized plants from every other population along the length of the Nekkies. (Here is where Levins could apply his ecogenetic concept!). What was most dramatic was finding some plants with flowers of the micans type (fig.9 in one population of hallii. I observed colour variants in other populations of hallii further to the south-east where the plants were primarily with bright yellow flowers. On revisiting the D. micans population just east of the Karoo Garden, I found that there were plants with the plain bright yellow flowers of hallii as well as pale rose-red to red flowered plants (figs.10, 11, 12).
The question now arises…”What else?” My own approach to taxonomy and plant identification, is that one has to consider not just the ecological associations of plants, which are mostly more difficult to describe accurately than any morphological property, but geographical distribution. Plant species are just like any other material phenomena. They are distributed in space, and they change with time. This is the determinant of their reality. Taxonomy and the decisions that are made are dependent on the material available to taxonomists and it is often simply not substantial enough to establish a practical reality in the application of their names. Looking further afield, I have three more populations of plants from the southern Worcester area namely at Jonaskop (figs13, 14), Lemoenpoort (figs 15,16) and Droogerivierberg (figs.17, 18,19, 20, 21) which fit the micans/hallii mould in terms of all but colour. The population of D. micans on the Droogerivierberg south of Worcester I thought to be fairly consistent in flower colour. It proved not to be and we found a wide range of colours, which extended to white and also included the more typical bi-colour thought to be the real thing. Curiously Mrs. Bolus also described D. leptum from Stormsvlei Pass as white with black staminodes. I have not been able to find it again, but it does seem, in the light of the distribution and variation of D. micans as I have discussed it here, that it is in fact also this species.
Outside of the Worcester area, there is a population north of the Langeberg mountains in the Keerom Dam area, which is characteristically micans (fig.22 – not that single images can represent the variation in the population). Still further afield is a population north-east of Montagu which suggests affinity with the populations of southern Worcester (fig.23).
Then there is Drosanthemum aureorubrum described from near Drew west of Swellendam. It has the characters of micans except in respect of colour. It is inclined to have flowers rather bronze-red-bronze in colour (fig.24) apparently without much local variation, and it should be noted that this variability can only be assessed in the field or by observation of plants mass propagated and cultivated. I can relate that population to three others, one from the middle Breede River Valley at Napky (fig.25), another from northwest of the Potberg at Potteberg Farm (fig.26) and the third from near the mouth of the Breede River at Infanta (fig.27). All well and good, except that the colour does not agree either with D. micans or D. hallii. Nearly everything else does.
The nail in the coffin of a narrow “character” driven taxonomy is a population northeast of the Bromberg Mountain at Stormsvlei in the Swellendam district. I had found these plants when without flower. Visiting the site in September/October when in flower was quite a revelation. There was a range of colours which included the bronze-red of Drew and the southern Worcester and Breede River populations (figs 28, 29). Very significant were a few plants with flowers coloured precisely the same as micans (fig.30). There were also plants with pure yellow flowers, but the yellow was distinctly on the golden-yellow side. I regret leaving out another dramatically varying population eat of Barrydale.
There is yet another “species” viz. D. lavisii, named by Mrs L. Bolus after Bishop Lavis who collected the original specimens ostensibly from between Struisbaai and Bredasdorp. Although Acocks also deposited a collection, purported to be this species, from Northumberland Point, I have looked for it there in vain. I have found three populations of what must be this species with its red flowers, from Napier (fig.31) from Swartjeskop (fig.32) northwest of Bredasdorp and from Soutkloof (fig.33) still further northwest. At first sight this did seem to be distinctive, but in summation I would opt for the geogenetic option. Looking at all the collections and attempting to relate them to the distribution of other plant species (AND their variants), I suggest that D. lavisii is another name for D. micans. I have seen a red-flowered Drosanthemum in the Bontebok Park at Swellendam (fig.34) and Van Jaarsveld and Pienaar report D. lavisii from the Goukou River in the Riversdale district (fig. 35), and these may be related to what Mrs. Bolus described as D. edwardsii from near George (figs. 36, 37, 38) If one considers the geographic aspect and notes the relationship of the distribution of D. micans and D. speciosum then one must perforce suspect that D. edwardsii is the eastern extension of D. micans. D. micans and D. speciosum do grow in very close association, although very seldom actually sharing habitat as they do on Jonaskop. My observation would be that the distribution of D. micans extends into the Swellendam and southern areas, whereas the distribution of D. speciosum is more karoid and it can be found eastward to Oudsthoorn. An apparently vicariant population near Uniondale has flowers which are golden yellow and with long pedicels (fig.39).
Thus we come to the point where we can ask what these names now mean. An aspect of exploration has been interaction with landowners and other members of the public. In this interaction use is made of names. Botanists (non-taxonomists) who have had reason to explore and report the plant species of the Overberg, have used the names lavisii and speciosum. They seemed not to have the capacity to assess if these identifications were right or wrong, and this is not because they were incompetent. It is essentially because from necessity and habituation they apply a species concept which has no reality. When one tries to convey to a lay-person what one is looking for, names clearly have either no meaning at all, or else are linked tightly to an image and association that the person may have derived from book or contact with someone else. It was most notable that in the Worcester/Robertson area, any red flowered mesem was linked to what I know as Drosanthemum speciosum and people found it difficult to conceive that there was a second species also with red-flowers. It proved well-nigh impossible to convey and communicate that either species could have different coloured flowers or that such variants were not different species. Fig. 40 demonstrate some of the variants in D. speciosum, also at Doogerivierberg, but while D. micans occurred in closer association with Witteberg Sandstanes, D. speciosum was on Dwyka Tillite.
A closing point is to refer again to Levin, where he is copied by Charles Craib in the magnificent work on The Grass Aloes of the South African Veld. Levin writes…”the eco-genetic species concept has utilitarian value, which is important from an operational concept.” The implication is that taxonomists have propounded a system of names which has little operational value. What then is the sense of taxonomy? Certainly this is what Craib conveys where he propounds a choice of a classification that serves the purpose of the user because the officially accepted system does not (meet the need of an informed user). The fact is that no system will have operational value while there is no universal definition in place, and common realization that a binomial system and names will never have any worthwhile reality unless there is a commonality about what names mean. Taxonomists have to start, with self-examination, to teach the user that names are for communication and have a meaning outside of the very narrow confines of obvious morphological difference, guess work based on limited material and knowledge, or idiosyncratic opinion. While it may be argued that the confused names I have quoted are directly related to the absence of a taxonomic revision of Drosanthemum since Mrs. Bolus last described her species, I use this example to reflect my long general experience with plant names and what people make of them.
Acknowledgment
I must acknowledge the association with Dr. H.E.K. Hartmann with whom I have been privileged to communicate over many years, and whose contribution to the knowledge and classification of the Mesembryanthemaceae parallels and possibly exceeds that of Mrs. L. Bolus. Also I would really like to express my appreciation to the many landowners who have taken us on trust and so kindly allowed us access to their property and plants. To list them all would occupy more space than this whole article but I must particularly acknowledge Messrs. Poffie and Hettie Conradie of Worcester (Droogerivierberg), Messrs. Jon and Cindy Webber of Cape Town (Klippoort, Stormsvlei) and Neil and Saartjie Neethling of Swellendam (Potteberg Farm). Jan and AnneLise Vlok very kindly sent me pictures from Riversdale and Mossel Bay. I do not know how to acknowledge Conservation authorities in terms of their permit system and how these are issued and monitored, nor the paranoid response one gets to the term “collecting”. There is in my opinion a total failure to make any distinction between collecting for knowledge gain and satisfaction of natural curiosity, intelligent plucking for financial gain, or crass exploitation that could lead to degradation of the environment, nor to balance this against doubtful control of development and concomitant destruction of natural vegetation by other agencies. A permit applicant seems to be regarded as a confessed reprobate with no sense or sagacity and an immediate threat to biological diversity. At least this is my impression of how I have been seen. Who wants to draw attention to themselves in this way? Steve Hammer kindly commented on the manuscript.
Reference Levin, Donald, A. 2000. The Origin, Expansion, and Demise of Plant Species. Oxford University Press.. ♦




























































New finds in Haworthia
Previously published Cact. Succ. J. (Los Angeles) 84(1): 41-50.
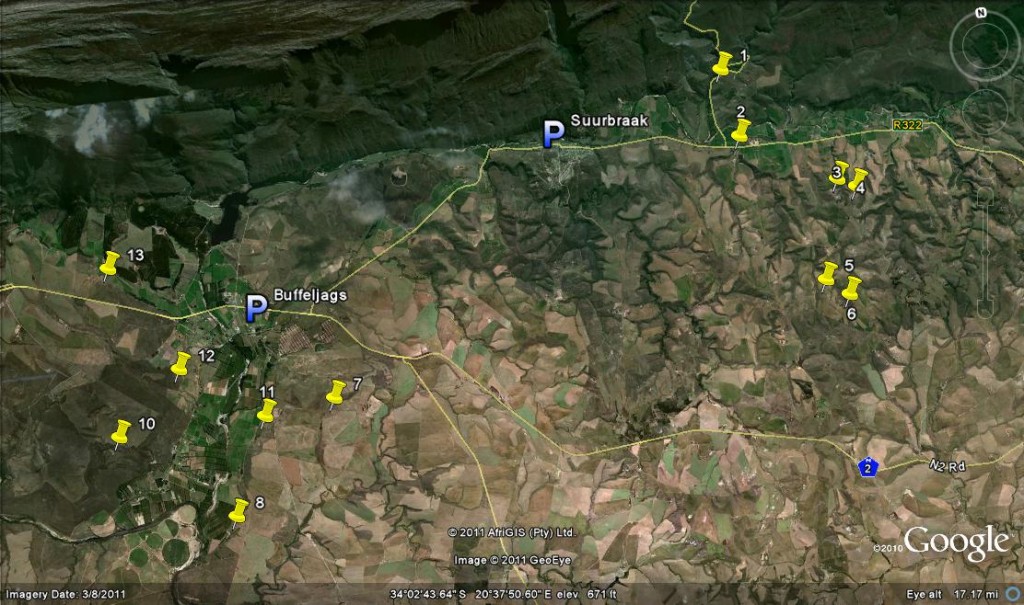
Map Legend – east of Swellendam.
1. JDV84/75 Haworthia retusa ‘turgida’.
2. MBB6666 H. retusa ‘nigra’↔ H. mirabilis.
3. MBB7898 H. retusa ‘nigra’.
4. MBB7899 H. retusa ‘nigra’.
5. MBB7897 H. retusa ‘nigra’.
6. MBB7896 H. retusa ‘nigra’.
7. MBB7871 H. mirabilis.
8. MBB7823 H. mirabilis.
9. MBB7909 H. mirabilis
10. MBB7805 H. mirabilis.
11. MBB7801 H. mutica ‘groenewaldii’.
12. MBB7886-7889 H. mutica ‘groenewaldii’, H. mirabilis, H. minima, H. marginata.
13. MBB7722 H. floribunda ‘major’
1. Haworthia marginata and H. minima
The Robustipedunculares is quite a distinctive group within the currently recognized genus. While the four species in the group are generally quite distinct, there are some remarkable complexities that rival that elsewhere in the genus. Recognizable and obvious hybrids are found between H. marginata, H. pumila and H. minima, but there are instances of whole populations that appear to consist of such in-between forms e.g. H. Xmortonii. I have speculated that perhaps rather than have just hybridized, the species have never ever really truly separated in the supposed evolutionary process. There is a vast body of variants that still link them in intimately as this piece will show.
I long ago observed that a small remnant population of ostensibly H. minima just south of Swellendam flowered in November as opposed to the general rule for the Robustupedunculares as late summer flowering. A vicarious population at Brandrivier north of the Langeberg (H. minima ‘opalina’) also flowers in November and both populations have fairly large and white flowers for the species.
I have recorded the normal bluish-green H. minima within the Bontebok Park at Swellendam as well as a very green variant. But in very recent exploration to the south-east we found an even more divergent group of plants that, while varying among the plants, seemed to be hybrids of H. marginata and H. minima (fig. 2 as a single sample and not representative of all). It was September and there were no signs of old or new flower spikes. Kobus Venter, who was present, remarked that the first plant seen was reminiscent of the plants once present south of Swellendam. The plants were large and in exposed situations even colored brownish as does H. pumila. No flowers were present and their color may have shown if H. pumila could have been directly involved at all, while it is essentially its distribution restricted eastwards from the Robertson Karoo by some 20km that reduce the possibility.
What makes the situation more interesting is that nearby was a population of H. marginata that was flowering and the flowers were also large and very white for the species (figs 3 & 4). Added to the fun was a smaller probable hybrid (fig. 5).
Differences and complexities like this do not really surprise me because it is what I have come to expect in my many wanderings in the field. The problem is that it certainly makes classification and any agreement on a set of names very difficult. I just accept it as a fact that plant species can exhibit greater differences between individuals of the same species than between individuals of different species, ridiculous as it may sound. This is because I perceive species as systems of individuals in populations with a very strong geographic component. To actually make a decision it is frequently necessary to determine just what else is growing in the vicinity in respect not only of the genus in question, but also that of other plants. Even the habitat factors need to be considered.
In the case of the plants pictured with this, the Bontebok Park terrain is mostly tertiary gravels, while the habitat we found the plants in was more recent riverine boulders. It is very curious that in the description of H. groenewaldii, the habitat is implicitly described as Ruens Silcrete. I do not think this is true. It is in the true Ferricrete – Silcrete that we found the next and it seems to be these differences in substrate geology that play a large role in generating variation and consequent controversy.




2. Haworthia retusa ‘nigra’
I first allied this element with H. mutica because this is how G.G. Smith referred to his collection from Kransriviermond south of Heidelberg. Since then there have been many new collections from which can be gathered that this population is a hybrid one between H. retusa ‘turgida’ and H. mirabilis. There is another collection north of this at Morning Star that appears to have the same parentage but also including H. floribunda. Then there are populations continuing up the Duiwenhoks and then Klip rivers to northwest of Heidelberg, a population between Heidelberg and Tradouw Pass further west and also a population at Goedverwagting south from there. Apart from the Morning Star population (February – March) these are all September/October flowering. There is a population at the southern entrance to Tradouw Pass of the same ilk that is February/March flowering. Then there is quite a distance between these known populations and a remarkable population at Buffeljags south of Tradouw Pass. It is remarkable for the fact that it is lauded as a new species viz. H. groenewaldii when I consider it to be generated from the interaction of H. mirabilis, H. mutica and H. floribunda. I attach no special importance to the fact that it flowers, contrary to H. mutica, in February – March. This is because I have observed many hybrids between patently different species despite a seasonal difference in flowering time.
To explore the realities of the situation we undertook two expeditions, one was to Buffeljags to explore west of H. groenewaldii and the other was to the Tradouw Pass area to explore H. retusa ‘nigra’. The first exploration yielded three populations of ‘groenewaldii’, which convince me that despite its flowering time as for H. marginata above, is simply H. mutica in another guise. I also think far too much is made of superficial and trivial differences that are as much characteristic of the variation in the one original population as they are within the four populations and for H. mutuca in its full sense. I consider it significant that H. mirabilis in its more normal non-retuse and dark green form is present in discrete populations both at and west of Buffeljags.
The second expedition was nearly as fruitful. It showed the Tradouw Pass population to be February/March flowering (see figs 6 & 7), while three new populations we discovered between there and the previous records in the easterly Heidelberg direction were September – October flowering. They link up to the populations elsewhere that I assign to H. retusa ‘nigra’. An additional find by Jannie Groenewald, for whom that H. mutica viz. H. groenewaldii, variant is named, also took us to a population of what is clearly H. mirabilis (see map) as I know it in its many disguises in the white kaolinic/bentonite clays of the silcrete – ferricrete inselbergs throughout the low-lying Southern Cape. There is unquestionably an overlap of characters between what I assign to H. mirabilis and H. retusa and I consider inarguable that H. mutica is a reflection of a shared gene pool.


What this demonstrates again, as does the Kiewietsvlakte populations between Heidelberg and Riversdale, that H. retusa and H. mirabilis are closely intertwined from east to west. There is an added complexity that H. floribunda is admixed along the northern populations and H. variegata along the southern. The admixture of the two species produces H. retusa ‘turgida’ and H. pygmaea in the east and H. mirabilis, H. mutica and H. retusa ‘turgida’ in the west. This is complicated by the other interactions along the northern and southern areas. In the Potberg area it appears that the genetic material of all five “species” is evident in the populations that I have seen there.
While I would like to explain the situation around H. groenewaldii I. Breuer that I interpret as a variant of H. mutica, this should be left for another occasion as too many images are required to support any argument. As it is, the issue of H. mutica ‘nigra’ occupies 29 pages and 79 illustrations in my book Haworthia Update Vol.2 pt 1:50, where all the above mentioned variants are discussed and illustrated. The naming of Haworthia is highly contentious because the species consist of aggregates of small fairly isolated populations that may differ to large or small degree. The populations are in turn also aggregates of plants that can all be identical from vegetative propagation, similar because of low genetic difference or very different from each other because of large genetic differences. Therefore figures 6-13 simply show just a sample of the variation within these four populations in the Tradouw Pass area. The plants vary quite considerably in size too and the one in Fig 8 is nearly 200mm diameter. I have added to the map locations of the only significant other populations that I know of in the area including H. retusa ‘turgida’ and H. floribunda ‘major’, excluding those within the Bontebok Park viz. H. minima, H. mirabilis and H. marginata.







Perhaps I should close by explaining that I have dropped the use of any rank below that of the species name. I simply am suggesting that we recognize the need for a trinomial system without the pretension of status, and more greatly honour the binomial as an entity of a greater significance than we may know. I do this because botany has no proper species definition and consequently species descriptions are just based on wild guesses about possible non-similarity and on the flimsiest of supposed character differences. The loosely used word “typical” is only truly useful in respect of the one plant dried as an almost unrecognizable specimen that is used to anchor the Latin name.
Acknowledgement. Any proper excursion into Haworthia territory always requires acknowledgement of landowners and I thank Jaap Viljoen and Jannie Groenewald for organizing that and for their company. I was also glad to have Kobus Venter along who had persuaded me to show him some of populations known to me on promise of new exploration.
Note.
Cross seasonal hybrids observed are-
H. retusa turgida X H. floribunda Blackdown, Heidelberg.
And also Platjieskop, Riversdale.
H. pygmaea X H. floribunda Coopert Siding, Albertinia.
H. mirabilis X H. retusa Soetmelksrivier, Riversdale
H. mirabilis X H. variegata Stoffelsriver, Swellendam.
References. I need to record that Harry Mays of Alsterworthia kindly undertook the non-profitable publication of 5 volumes of Updates (2-6) between 2006 and 2009. Vol. 1 was published by Umdaus in 2001. These volumes were the product of research to validate or correct what appeared as a formal revision in Haworthia Revisited, published by Umdaus in 1999. The description of H. groenewaldii appears in Alsterworthia 11.2:15 (2011). ♦
What do collectors need?
Previously published in Alsterworthia International Volume 12, Issue 2. July 2012.
I ask this question because too often the views of the collector are espoused as an excuse or defense for some or other argument about classification. It has often been said to me that collectors are not interested in taxonomy and they are at the most, happy just to have a name. This argument does not impress me because as a society we have a trust and a belief in science and whatever is written, outside of fiction, should seriously address the truth. It should not matter what the reader may want to make of the product other than that the reader may just by chance really want to know and understand something. On reflection, one writes for the reader who must surely be reading because they want to know something, and names are the key to the “something”?
This is why I have responded to reviews of my writing that have been published at various times. I have written as a communication and am glad to know what the reception or rejection has been. Recently Steven Hammer wrote what is listed under the title of “Book Review” comment on a recent book by Ingo Breuer and of Update Vol. 6 by me. It is a wonderful piece of prose and worth every bit of reading and appreciation, but it does not pass as a Review. Or does it? I feel that it has a few mistakes as well as passing over the very real differences between the two publications. So, I wrote a response in the way I treat any publication as an invitation to think and form an opinion; and express it. Passing a draft of this response to a competent observer, I got this reply …”Fortunately, there is little expectation of a review. The point is: was the review positive or negative? Did the reader learn something and gain deeper appreciation, or not? Will they buy the book, or did the review satisfy their curiosity? For most readers, the details are unimportant, as much as you may hate this very concept.”
Why I should hate the concept of most readers regarding the questions of detail unimportant I do not know. But I do think the accuracy, in respect of detail or general, is very important. What my commentator was implying is that the review met the requirements that he was suggesting, and he added that my response was “nit-picking” and would only be seen as criticism of someone who is widely held in high esteem. The fact is that Steven Hammer is also held in very high esteem by me and I am so glad to be able to say that he expressed to me personally that his “review’ was rather a literary fantasy. What Steven does comment on is a view of the needs of collectors. That they care little about schemes of classification and that labels are necessary irritants. I do not question the truth of this view. But would not accept that this is a justification for the imposition of just any kind of scheme because that is what a writer wishes to propound for reasons of his /her own.
These then are the points I made in my response that I think Steven should have addressed. The ‘mistakes’ are … a). The Audensberg population was actually shown to me by Elsie Esterhuizen many years ago and it is not the place where any haworthiophile would ordinarily look for plants. b). The reference to Drosanthemum bellum is odd because Steven describes this as a “niche-sensitive species”. This “species” is at the heart of a very long and detailed story of Drosanthemum micans that I once wrote and lies unpublished. I would surely have used this as an example of the way in which botanical science has also failed us. D. bellum is a pink flowered variant in a much-localized population of D. micans that also has white, purple and red variants. This tiny population sits among a larger widespread population of yellow flowered variants that go by the name of D. hallii. This is turn has variants that include the typical bi-colored flower of the older D. micans that is common north of Worcester. Further variants occur north-east of Montagu, to Oudtshoorn and then south to Mossel Bay (D. edwardsii) back west to Bredasdorp, (D. lavisii, D. aureopurpureum and D. croceum?). The problem here is the failure to establish what is meant by “species”. To refer to D. bellum as a species is a misconstrual of science, or an example of the liberties that are taken with Latin names – botanists and collectors alike. c). Chameleons. Wonderful words of Steven’s, but not quite complete. The story about chameleons’ parallels that of the very low non-tech problem of impossible identification even when there are heaps of “characters” to use. It fortuitously exposes the probability that we are being led up a garden path by high-tech. I have used chameleons in the same way that I studied Oxalis ultimately demonstrating that species are complex systems of variables! d). Kaboega is not the only area I know exceptionally well and it also figures in practically all the other volumes of Updates of which there are five. I doubt if these have ever had much coverage, but they are an account of my voyage of exploration and discovery that is the concern of mine in respect of omission. In the Updates I discuss the populations and their variants as they occur at many different places and show this impact on the application and use of Latin binomials. There is a prevailing misconception that this is only a problem within Haworthia. I show that this is not so. I also make several references to the fact that Haworthia is by no means an integral single genus and that the nature of genera in the Alooideae needs proper attention based on a lot more than the fact that the Haworthia subgenera have small flowers. e.) “Shaggy dog” for H. mirabilis Ballyfar is not a name I coined but Steven himself.
I do think the omission is in the comparison of the books where there is in fact none. My Update Volumes revolve around the way that science has let us down to the extent that any pretender can take up the mantle of taxonomic expert. Botany provides no species definition and hence the Latin binomial is not required to carry any meaning other than a guise of authority. Whatever collectors may require has no import whatsoever in a process of classifying plants as biological entities. They are focal points for the collection and storage of knowledge indicated by Latin binomials and these are not simply and only intended as labels. Even I recognized this as a child when I wanted to know what the plant actually was that my father called H. chalwinii, and where it came from. Every collector who refers to names at all surely expects and believes that there is some mystic or real knowledge associated with the names he is given and uses. It is an injustice to any collector to coin Latin names outside of the context of science where the collector is entitled to believe they belong.
The only predicable thing about Breuer’s system, which is a watered down version of a much more focused and detailed one by Hayashii, is that there are going to be a lot more names. This is not only when someone else climbs the Audensberg, or recollects the Sandhills population, or drifts across into the Heatley Peak area. Throughout the Updates, I warn and demonstrate that character fixated taxonomy may be very misleading. Vavilov was a Russian botanist who pointed out that variations in a genus may be expected in all the members of that genus. Species are therefore to be seen as systems that are natural assemblages of plants that can be associated in respect of ALL the forces, factors and features that generate them – not propagules of single clones that fill availability lists and price catalogues. Drosanthemum bellum is just such an example of how Latin binomial names are used to describe variation within species, rather than to properly organize the basic entities that make up the entire living system.
I have, even in the Updates, shown how the watchdogs of science let us down. I have tried to communicate my experience and observations to a wider and expectedly interested audience. This, in the hopes that it would lead to greater understanding and comprehension of the problems of finding names as the backbone of communication, appreciation and understanding. It is a huge disappointment to me that I have achieved very little other than to grow wiser myself. One of my many critics makes a show of taking up middle ground between me and other Haworthia taxonomists. My response is that taking up middle ground between myself and the ignorant is not going to be very productive. In the first place there is not much space there as I am quite aware of my intellectual limitations. In the second place I have not actually been all that certain that my overview is entirely correct. Despite being credited with a lot of field work (and no good sense to go with it) I am extraordinarily aware of how much I have not seen. This adds to my discomfort as I see a proliferation of new names, gaily forgetting the multitude that I moved aside in my Revision. These are often based on propagules from my own collections (concealed by the creation of new collecting numbers that are not mine). I recognize that the only predictive element in this kind of science is that we can expect many more Latin binomials in a collector driven system rather than one of botanical science. So indeed I see no change from the failed methodology of von Poellnitz, Smith and Scott.
There is no comparison at all between the two books that a true review might have suggested. One book (Breuer) is a collector’s guide to a limited range of variants (albeit 336?), while the other (Bayer) is an account of a very wide range of variants and a hypothesis (not a statement) of how they are related. The latter also throws some light on the universal quandary knowledgeable observers soon come to experience viz. Elton Roberts in the same edition of the Journal where he questions the identity of Mammilaria lasiacantha. The problem he has is a classification fixated on superficial small differences rather than one based on the realities of the variation that should be expected in any system arising from, and actively responding to, differences related to time and space. The essence of science is that an experimental method is applied to a sample and repeated, the result will be the same. If everyone is coming up with a different plant classification, it should occur to us that there is something wrong with the method and perhaps also the hypothesis that is being tested.
I am curious why my commentator dismissed all the above as “nit-picking” when myself I feel that they deal with the most significant elements of writing at all. Especially, they touch on the very core of why we even classify and name plants in the first place. My response should not be seen as a criticism of my commentator, or of Steven, a remarkable man who is also very dear to me. I also respect him enormously for his empathy with plants because if there is anyone who projects my view that this is a conscious creation all the way down to its rocks, it is him. There is surely purpose in creation if only that we should seek and find what that is. ♦
Righting some misconceptions
The article by Gerhard Marx in Haworthiad 26.2:41 is really excellent. There is however some behind the scenes innuendo as well as repeated use of words like ”ignored” and “neglected” that need attention. There are furthermore comments on “substantially different and unique characters”, “forcing” elements into synonymy and use of words like “truthfully” and “knowledge of the genus” that distorts a reality that is historically under stress. This is totally unnecessary in an article that otherwise contributes so much in the real area of interest and information.
What we first could deal with is this vexing question of what science is and who is a scientist? In the issue of Haworthiad the editor feels it necessary to state that he is not a scientists and both Al Laius and Stirling Baker imply that there are things that the uninformed dare not comment on. Let me make it clear that most of the writing and classification of Haworthia has been done by authors with no formal training in biology whatsoever. I could fill the whole of an issue with detail of how trained and experienced botanists (scientists) have made mistakes in their small contribution to our “knowledge of the genus”, or simply confounded it.
What the Gerhard Marx’s article actually does is ignore history and written knowledge of the genus. He does profile himself on Facebook as a “succulent botanist” but I doubt if this meets the South African Association of Botanists requirements for membership. Many taxonomists have not been scientists at all, and one cannot fault the enormous contribution that most ordinary people (education-wise) have made to botany. So who am I then? With an MSc and a thesis on morphology and taxonomy, am I a scientist? Does training and a formal qualification mean anything at all? Am I really just another writer? Perhaps not. But…
My first bone of contention is the use of the word “ignored”. This is because it is combined with the notion of unknown transitional elements. This is plainly misleading and incorrect. von Poellnitz described H. mirabilis ‘beukmannii’ as a variety of H. emelyae probably as the first clue to some sort of transition between elements south and north of the Langeberg and Outeniquaberg. von Poellnitz cites several specimens from north of the Langeberg that he confused with H. pygmaea and what I later recognize as the totality of H. mirabilis. As long ago as 1976 I published a map in Excelsa, replicated in my Updates, to speculate on the transition of the southern retusoids into the Little Karoo as far as Steytlerville. In a recent treatise available to all I explained the remarkable similarity that I perceived in H. pygmaea in the Herbertsdale area to H. emelyae a relatively short distance away north of the Gouritz Gorge. Many years ago and lost in my writing is the extraordinary similarity of H. emelyae ‘major’ and H. mirabilis ‘paradoxa’. While surely there must be a record there too of the extraordinary similarities that I observed of H. emelyae ‘multifolia’ to H. mirabilis ‘sublineata’ as well as to H. mirabilis ‘heidelbergensis’ and even to H. rossouwii.
As an aside I must just draw attention to Al Laius’s comments on what he refers to as H. heidelbergensis and H. magnifica. This is because Marx also uses names like átrofusca’, ‘magnifica’ and ‘mirabilis’ with no appreciation of the realities of these names. This brings me to the second bone of contention – the one of “neglect”. One can only do so much, and if Marx can concede that then he can approach this from a more objective angle. I have been fairly limited by opportunity and finance apart from the enormity of the task, but I can and have pointed to a multitude of situations that are no different from the area in the Little Karoo that Marx has easy access to. I have not felt it necessary to venture there where activities of collectors and others have made it too often to ask the indulgence of landowners. Also, when there is just so much else to do. But one of the situations I must refer to is that of the “species” H. groenewaldii, the description of which was also instigated by Marx. The explanation of this taxonomic deviant occupies almost all of Haworthia Update 7 that is available in hardcopy and also on the internet. This explains some of the aberrations that filter through to Marx’s article.
Does that bring us to the question of “substantially different and unique characters”? My MSc thesis had as the subject the nature and significance of complex morphological structure – albeit in entomology. So I actually do know a little bit about characters. I also know a little bit about the use of DNA strings as character sources. More practically is the work that I have done in respect of characters in Asclepiads, in Drosanthemum, especially in Oxalis and even in chameleons. A scientist unfortunately cannot restrict him- or her-self to what they think are the realities in one small area. There is far more to life than Haworthia and it does not have any special problems. There are any number of substantially differences and unique characters even in individual plants. Marx throws this all away with a rather mixed final sentence and reference at the end of it to a “very consistent set of measurements”. Stephen Gould once said that the strongest statement that one could make in biology was “hardly ever”. A consistent set of measurements in biology is spelled out in the science of statistics viz. biometry. Perhaps that article by Loucka and myself dealing with the statistics of H. mirabilis ‘sublineata’ needs to be unearthed. Some variation in Haworthia mirabilis var. sublineata
So this takes me to the other bones of contention viz. “forcing” “truthfully” and “knowledge of the genus”. I have written elsewhere that the taxonomic system is at fault, and I will not even try to defend that simple statement. It is. One of the difficulties is that things that are neither here nor there have to be unequivocally assigned to a taxon. If this is coercion as Marx implies, then he is unaware of a very self-evident truth. There is no place for the chemical equilibrium equation or the subtle variants that Marx even describes. The sorry fact is that formal botany and the ICBN does not actually provide for “aggregates” and this is just a ploy to evade the issue of the definition of “species” and what it truly means. Manning as a professional botanist needs more than ordinary opinion, in a very small field of interest, on which to base a formal list of names for a national catalogue of 23000 names. To treat each genus in that list on the basis that Breuer and Hayashi have done would be unthinkable.
In closing we have this fudging statement… ”Haworthia will remain a taxonomical argument problem for many years to come”. It surely will when writers, editors and readers prefer the mileage they get from disagreement instead of properly informing themselves and working actively towards a common goal with “truth” as a commitment rather than as a word to discount what is already suggested. ♦
Comments on H. marxii
In An honorary Ariocarpus in Africa – Notes and updated information regarding Haworthia marxii (Alsterworthia Intenational Volume 12, Issue 2) Gerhard Marx and S.D. Gildehuys respond to the inclusion of H. marxii under H. emelyae by Manning and Bayer in the Rationalized List of Names.
The reason for my dumping H. marxi was purely political as I explained elsewhere. It is just my opinion that there were and are shortcomings in the author’s whole approach to names in Haworthia. So I expected some attempt at vindicating themselves like this. I have no problems with considering H. marxii as a species. I would simply prefer that it remains an element that desperately requires an explanation.
My problem is with statements like this …”Typical H. archeri can be found only … at Viskuil (JDV89/62)”. I described this species and from where it occurs at Ghaapkop and at Baviaans Station just east of there (and Viskuil is just a few miles further east). I later placed it with H. marumiana because of several other oddities widespread and also because of the very wide distribution and variability of H. marumiana. I have since found it also at Lospersberg. Marx has previously been on at me about the relation of H. archeri to H. marumiana. I did not realize that he was unaware of what actually constituted the knowledge of either species.
There is also mention of a collection of PVB1405 as from 20km SE of Rooiberg Pass. This is a bit of a mystery to me as I thought I had noted this collection of PVB from east of the Floriskraal Dam and was for a long time the only possessor of the single plant in cultivation. This collection is cited in my Revision as are the two “archeri” collections noted above. I am sure I noted this collection 40km ESE Laingsburg. It is a mystery because it is north of the Witteberg and Swartberg and is tied in with another population that Bruyns recorded at Baviaanskloof. Every indication is that it is connected to a population of mine at Scholtzkloof, Prince Albert; and so with things that I pondered may be a marumiana connection i.e. ‘var. viridis, or even connected to monticola or more probably, H. vlokii.
Another statement I must make is about the significance of flowering time, as the evidence is that it is not as meaningful as the authors imply. There are hybrids and population evidently arising from hybrids in these interactions – retusa/mirabilis, mutica/mirabilis, floribunda/pygmaea, floribunda/retusa,and herbacea/reticulata. The latter is not across a seasonal flowering difference but the others are.
The large gap between Montagu and Rooinek Pass is reduced somewhat by the old populations of H. mirabilis northeast of Montagu at Dobbelaarskloof and by the new population recorded near the Pienaarskloof Dam. Also relevant is the distribution of H. pumila. So maybe exploration has simply been inadequate. I was and am fully aware of the distance issue.
Regarding flower morphology. Similarities in the flowers of otherwise very different things, is just as significant as differences in the flowers of things that may in fact be very similar. So these small differences should not be punted without far more attention been given to what actually constitutes difference. The few pictures of the flowers that are provided are hopelessly inadequate to base any opinion on. The ultimate issue is that the authors are using idiom, method and logic that have been in service since before von Poellnitz, Smith, Scott and the rest of us. It has not worked. It is not going to resolve the complications we can find in situations like between say H. mirabilis and H. retusa, or H. arachnoidea and H. mucronata, or H. cooperi and H. cymbiformis, or H. cooperi and H. bolusii var.blackbeardiana. To name some of the more obvious interacting species or elements if we do not know what species are. ♦
Haworthia maculata ↔ Haworthia pubescens, MBB8002 Cilmor
In Haworthia Update Vol 9 there is a report of a population MBB7997 identified as Haworthia pubescens from north of the Cilmor wine cellary. This is approximately 2-3km southwest of the type locality for the species. I noted that the plants have less spinuliferous leaf surfaces and there is a degree of surface translucens and maculation (spotting). I also presented 3 pictures of MBB7271 of what I identified as H. maculata from south of the Cilmor cellar. When I first visited this locality I had no problem identifying the few plants I saw as H. maculata on account of their marked spotting. However, on a recent visit we struggled to find plants at all and the few plants we found were too embedded in rock cracks to make any worthwhile identification. So we revisited the site to explore more extensively and located a large number of plants higher up and slightly west of our first sightings. These plants are illustrated here. They incline more to H. maculata than the plants at MB7997 and I have accessioned the population as MBB8002. There is the usual expected large variation in respect of superficial and observable characters. The plants can be proliferous and cluster, more so than at MBB7997. Similarly the leaves can have more translucens and even less spinuliferousness of the surfaces. Some plants have few and quite thick swollen leaves while others may have more and very slender pointed leaves. I have not observed the flowers and really do not expect them to make any difference to the problematic classification of populations that again are neither here nor there in a narrow concept of species. H. herbacea occurs at all four geographic positions at a radius of about 2km. At the brickfield to the northwest as well as just northeast of the Brandvlei Dam wall it is evident to me that there is a transition between H. maculata and H. herbacea. I did report the known distribution of H. maculata in Update 9. While there is no suitable habitat between Die Nekkies hills at the Brandvlei Dam and the Audensburg or Kanetvlei, there is unexplored suitable habitat southwards to Moddergat and Hammansberg. There is no evidence of H. maculata eastwards to where H. reticulata is known about 15km east on Ribbokkop. Westwards no Haworthia is known although G.J. Payne did inform me that he had observed plants in the hills immediately southwest of the Dam at the now submerged hot spring in the Brandvlei prison area.


The submitted pictures include two views. View 1 is looking north of east across the Breede River to the Sandberg where H. pubescens occurs. Its full occurrence on those low hills is not known and this I will explore soon. View 2 is looking eastwards looking at a Dwyka Tillite hill across the river in the upper right. We found no Haworthia on that hill although both H. pumila and H. herbacea are present on the smaller rise to the right and behind it – also Dwyka. H.herbacea is very abundant on a Dwyka tillite hill about 10km to the south. The corresponding hill on the left is Ribbokkop where H. herbaea, H. reticulata and hybrids are present, and H. arachnoidea also occurs. The limits of H. mirabilis are the higher hills in the background viz. Rooiberg, Gemsbokberg and those are Witteberg sandstones. ♦
Addendum Haworthia pubescens MBB8011, SW Sandberg
I need to point out that there is a still earlier article which covers Haworthia maculata (Haworthia maculata <–> Haworthia pubescens) that lays the basis for this discussion. In that article I note the position of the Sandberg to Cilmor and DeWetsberg and intended to include the Sandberg H. pubescens in that article. We could not get landowner contact and so that fell away. However, this problem was overcome and we first explored a Dwyka Tillite outcrop southeast of Sandberg. There is a vast accumulation of windblown sand on the first hill and we saw no Haworthia. There is a smaller hill further to the southeast that is also Dwyka and erosion exceeds wind deposition so smaller non-geophytes do quite well. We found both H. herbacea (see fig.1 MBB8014) and H. pumila there. From there we went to the southernmost point of the Sandberg. A misjudgment landed our vehicle in mud and the drama to get out limited the time we had to explore. We found a lone H. herbacea (fig. 2 MBB8012). Returning a week later we approached the Sandberg from the southwest, and almost immediately on reaching the top we found H. pubescent. Fig. 3 is a view towards Cilmor and DeWetsberg where the plants appear to be intermediate H. pubescens↔H. maculata. The picture is useful to get some idea of the role of geographic and geological considerations. The high mountains in the background are Table Mountain Sandstone and no Haworthia is known there. I am not certain that this is true and G J Payne did tell me that he had seen plants on the extreme lower right and south of the Brandvlei Dam. But also on the absolute distant and absolute left, is the Riviersonderend Mt. That is also TMS. The deep Wolfkloof Valley behind that is the locality for the much unexpected H. herbacea ‘lupula’. (These inverted single commas are not entirely necessary but I use them to underscore my informal use of names that have less reality. The var. lupula is real). The mountains ahead of that last line are Hammansberg on the left and the Moddergat to the right. Between there and DeWetsberg has not so far turned up Haworthia, but this is an exploration problem. Behind the DeWetsberg is also underexplored. H. herbacea does occur between DeWetsberg and behind the mountains on the low right just in the picture and also east of the brickfield out further right. H. maculata is only known in this area along the Nekkies north (further to the right) of the Brandvlei Dam just visible in the picture.



Prior to this exploration H. pubescens was to me only known from the northern part of the Sandberg that lies south of a road going eastwards to Eilandia between Worcester and Robertson. Here H. herbacea does occur on the lower northwest warm slopes. H. pubescens seems to occur only on the upper two ridges and H. herbacea is not known to intermingle with it. This is all Witteberg Sandstone that as a formation overlies Bokkeveld Shale and underlies TMS.
Coming back to the southwest corner of the Sandberg where we found H. pubescens. The plants seem very similar to the species as it occurs to the north. They were very cryptic and often in shady rock retreats where they were really hard to see. It was mid- to late-morning when we were there and the plants were not going to be better exposed as the sun moved further west. Although there was very suitable well-drained habitat lower down on the shaded east slopes, there were no plants and I speculate that this may be because the plants may need the cooling effect of wind movement up on the ridge. The pictures tell the story of variability in respect of a whole range of leaf and rosette characters.
It is worth noting fig. 43 of the dead remains of a plant under a clump of restioid. It seems that seedling survival is closely coupled to early protection giving rise to the concept of nurse-plants. Plants are often very difficult to find because they are so hidden beneath accompanying vegetation. But they do need light and the dynamics of vegetation growth and densification must have quite a big impact on the ageing and survival of plants. It raises again the question of how long do the plants live? For plants like Aloe ferox and A. dichotoma I do have a real experience of a lower limit of about 35 years and a top limit in the several hundred. In the field, the plants seem comfortably ageless.
The really interesting part is this. While I was busy tediously cleaning a plant to photograph it, Daphne called to me to come and see a lighter green plant she had seen. Moving in that direction I saw a plant that registered as H. herbacea but with some hesitation and doubt (see fig.4). I then went to see what Daphne had observed. They seemed less obviously H. herbacea but that seemed to be a logical and conservative opinion (see figs. 5-11). That was until Daphne found two adjacent rosettes at the foot of a restioid clump that left me in no doubt that they were hybrid H. pubescens/maculate (see figs 9-12). These were in bud whereas H. pubescens plants showed no sign of impending flowering. Note the buds are less well developed than MBB8014 further east, even if possibly insignificant. Going back to the other plants we confirmed my doubts. They were a lighter colour and apparently softer texture that we would have expected in H. herbacea. These were the only plants we saw in a space bridging the occurrences of plants of H. pubescens.











































12-46 MBB8013 H. pubescens, SW Sandberg.
We did not explore the western slopes where habitat would have been more suitable for H. herbacea and I expect it does occur there. What puzzles me is that so frequently have I found very distinctive hybrids between species in close proximity and very seldom where the species are some distance from one another. I cannot say I have ever found a hybrid in the clear absence of both parents. The example of Astroworthia bicarinata at Lemoenkloof, east of Barrydale, may be an exception where only Astroloba corrugata (syn A. muricata, A. aspera) is present but H. pumila apparently not. Hybridization is thought to be an important element in the “evolution” of new species. I doubt this as it is quite evident that separation into two species is a pre-requirement. If new species have evolved in Haworthia by hybridization, how did they evolve as such in the first place? The answer to me lies in the continuities between populations. I observe, and have experienced of expected continuity between populations. While the Cilmor populations are thought to be H. pubescens ↔ maculata it cannot be said anymore that they are hybrid, or populations where the morph or drift to discrete elements has not reached a conclusion. The latter is more likely. As there is already apparent geographic continuity of H. maculata and H. herbacea, I was expecting some evidence of a similar relationship between H. herbacea and H. herbacea. So here it is. Hybridization as a factor in speciation in Haworthia does not seem to very likely. It confirms for me that there is a fractal “chaotic” order to species in Haworthia and the reality is that a view of many truly discrete species is a fabrication and a very ill-considered view.
Acknowledgement
We are always greeted with such kindness and helpfulness that we might have expected this from the Sandberg landowners too. It came in no small measure. Driaan Griesel was most enthusiastic and interested and also helped us with extracting our vehicle from the mud on the one occasion, and then jump-starting it after a flat battery on the second. Our imposition did not so much as touch his view of the day.
[Ed.] Bruce made another visit to Southwest Sandberg on 9 December 2012 and includes the following flower pictures. He makes this comment; personal correspondence 27 December 2012.
I am actually not sure at all about flowering time now. I used to be quite sure of being able to collect seed of pubescens mid-Nov. But I observed at Humansdorp that gordoniana peak flowering could be out by 6 weeks. In any case the plants can produce successive spikes so one can get delayed flowering and added to that energy in the first or the second flower set. I know mirabilis at MacGregor can flower from Nov. thru to March while at Montagu mirabilis can flower as late as April/May. Retusa and geraldii are quite happy to produce flowers in either Spring or Summer and Kobus observed that splendens did that too. Maculata can flower from Sept. thru to late Dec. And each population does its own thing.







































































.♦
Genetic history – the place in Haworthia classification.
Preamble: Maybe the difficulties and strife in Haworthia classification is that we do not know what it is that taxonomists do. Taxonomy is the academic discipline of defining groups of biological organisms on the basis of shared characteristics and giving names to those groups. The scientists that do taxonomy are called taxonomists. The names taxonomists give to species don’t just tell us what they are called, but also tell us about how they are related to one another. In the following article Bruce Bayer shares his views that Haworthia taxonomy should be about more than identification and descriptions, rather the interpretation of the characters that reveal genetic relationships and evolutionary pathways. ~ Lawrence Loucka, 7 September 2012
Genetic history – the place in Haworthia classification.
By M. B. Bayer
This is a huge subject and I cannot by aptitude or knowledge attempt to cover it fully. However, it is so basic to plant classification and Latin naming that we all need to have some background understanding. The entire object of classification boils down to arranging living (and once living things) in a scheme that follows the theory of an evolution from simple life forms to the present array of creatures. This is because it is thought to be the logical way in which knowledge of the said creatures should be organised i.e. according to the way in which they traveled the creative way. All classification is simply a way of organising information so that one can generalise from the singular to the plural.
Whether one believes in such a progressive process or in a spontaneous divine creative event does not matter. All the evidence points to change of some kind and if we call it evolution does not alter anything. It is argued that there is in fact no direct proof that, say, man evolved from a primitive primate. That may well be so, but there are many fossil primates that are speculated to fill that gap. The case is that evolution is a scientific hypothesis and science does not, or cannot claim, that this is proven. A scientific hypothesis simple stands as a possibility or probability of something known. Such a hypothesis can only be proven untrue, and then it is replaced with a new hypothesis that stands as the accepted truth.
Whatever, the ultimate truth of the matter, classification in science is directed at reflecting how the different life forms are related to each other in respect of change? In the acceptance of evolution the process of change is the genetic history of the organisms and is referred to as the phylogeny. It is often depicted as a branching tree with the branches originating low on the trunk as ancient origin, to terminal branching for recent.
My experience with classification comes with education and qualification in entomology (insects). It so happens in insects (and higher animals) that there are many characters and structures that can be used to examine the process of change and modification that might go with evolution (change). The word “homology” is used. So, say, the wing of a bird is likened to the forelegs of a dog – they are said to be homologous structures. The change in structure from that of a segmented worm (eg. Onycophora) to say, a wasp, can be tracked through a whole range of insects. The head of a wasp is seen to be derived from 6 primitive body segments, the thorax from three, and the abdomen from several. There are muscles and nerve endings and sub-segments that are all evidence of these connections. In entomology, there was a stage when a qualification in insect classification required the student to also present a phylogenetic scheme to explain relationships relative to an origin. This all based on morphological characters, their homology and their interpretation as primitive or advanced. What is really odd is that too often a classification comes to deny the variability that a concept of selection and adaptation that evolution rests on, is denied.
In plants there is no luxury of musculature, or nervature or even detailed bone or segmented structure on which to base good homology. Botanists are therefore very hard-pressed to properly derive phylogenies or explain this genetic history. Nevertheless a very serious and honest effort is done to do this. The process of cladistics was derived from entomology (Hennig) and became the standard for the student in botanical classification. Students were required to evaluate characters on a basis of primitive and advanced, and construct cladograms that illustrated a hypothesized process of evolution. The advantages of the method over conventional decision making were that it is said to be an objective way of arriving at a conclusion rather than personal subjective decision making. With the complaints against my use of words and language, it is worth noting the remarks of a leading botanist who said that the methodology of cladistics was just the use of a new language that did nothing more than strain good-will. Agreed.
With the arrival of DNA sequencing, a whole new ball-game was created with each amino-acid base pair representing a character. So DNA sequencing has become the modern standard for the construction of phylogenies and genetic history – the phylograms. So much so that the article in Taxon 56:645-648, 2007 by A.B. van Wyk argues that the phylogram represents the process of phylogeny as the genetic history as opposed to the cladogram that attempts to construct the genetic history. I personally think that this is intellectual wizardry and the literature references in the Taxon article only expose the mystery that surrounds classification. It takes classification wholly out of the reach of any but a polymath. Basic to the whole issue is that scientists still have not reached any certainty about what a species is. The theory of evolution is not disproved. Van Wyk has said
Plant taxonomy, despite all its impressive achievements towards phylogenetic reconstruction, will then risk being denoted as yet another ivory tower science – a pursuit disconnected from the practical concerns and needs of everyday life, esoteric, over-specialized, its classifications of little practical use to the majority of end-users”.
It sounds very impressive, but I weep for the fuel that it adds to the fire of ignorance.
What truly appears to be the case to me is this. To construct a cladogram, the taxonomist needs to be very familiar with the plants and have a good understanding of the characters being used in the process; adjudging them according to some scale of advanced or primitive. The DNA sequencing method requires no knowledge of the plants at all and a phyllogram simply follows a statistical analysis of equivalence of the base pairs. What I see is DNA derived products that so far tell me no more than I already know about the genera, and nothing at all about the species other than some serious mismatches.
I argue for a species definition, not as some vague statement that species are things into which a genus can be divided, or simply a list of names under a Latin genus heading, or the zoological concept of closed breeding systems. My definition drawn from a lifetime of interest, observation and experience in things material as well as esoteric, is that the creation is a conscious one. This is not a new idea of mine. The conflict between religion and science is that the one maintains that creationism followed some sort of seven day event as in the book of Genesis. Whereas science (as we practice it today), has its origins in the quest for freedom from any such religious belief system. Whether creation began as a big bang, and complete or incomplete does not matter to me. We are faced with natural phenomena in the present that we can observe have changed and are changing. I do not think either the cladogram or the phylogram is anywhere near good enough as an answer, because they are two-dimensional diagrams that do not adequately portray the realities of time and space. Not only that, a three dimensional improved tree model will not show how fast things change within a time span. My model of the species tree is a fractal model that suggests species in a process of “chaotic” order. By the word fractal I mean the endless variation that seems to have no order. While I insist that the concept of a species of Haworthia must mean the same as the concept of a species of insect or frog, or bird or mammal, I do not mean that each species of Haworthia has the same branch clarity on any tree depicting relationships.
What I do not know is how fast populations might change in the process of genetic drift i.e. move off in a direction determined just by the local genetic content. Neither do I know how much nor how frequently interchanging of genetic material between populations occurs and how this is affected by distance between them. In the field there is a very distinct flow of difference (or similarity) with geographic spread. I have repeatedly found that with widely separated known populations, new populations between them are predictably intermediate. It does not seem entirely rational that there are not more random isolated individuals. My experience with hybrids is that they are very local to the mother plants while any populations tend to be very confined and local. What is odd is that hybrids may be found despite great differences in flowering times of the parent species.
The reality in Haworthia, however we define species, is that there are populations that are neither one taxon nor another. If the classification were to present a basic requirement that ALL populations known and unknown must fit the model, then definitely we have to consider that there are considerably less species than other writers are prepared to consider. One can continue building a classification from the top in which each new population is simply described as something new and given a Latin binomial. This process was suited to the origins of botanical exploration as well as nomenclature. It does make it a lot easier for collectors and the exciting process of always novelties for the collector. Alternatively one can decide that classification is by this stage a fully predictive one and one that must primarily suit the requirements of botany. If only botanical classification was more professionally and rationally based. Sampling is very extensive and a classification based on the known, is predictive. Personally I am very comfortable that I have achieved this in large measure – not entirely, but close enough and a lot closer than some critics will concede. With all that said and done, it is just a sad fact that formal classification is too often undertaken by people who have a need. That need may satisfy them within the limits of their personal experience and knowledge, with an uncomfortable disregard for the depth of knowledge elsewhere.
Reference: Readers can refer to the article in Haworthia Update Vol.2:149 (2006) “Nectar sugars in the Alooid minor genera and a need for another model”.
♦
Haworthia mutica (groenewaldii) and its twisted leaves.
In this article Bruce Bayer responds to the notion that apparent leaf twist arrangement is a defining characteristic and further explains his disagreement in recognizing the Buffeljags plants as a new species. (Also see Haworthia flowers – some comments as a character source, Volume 7, Chapter 4:- What is typical Haworthia mutica?, and Volume 7, Chapter 2:- Further exploration in Haworthia. Further to finale.)
The species definition Bayer uses is that populations are fractal and DNA is governed by mathematical non-linearity. What does that mean? We have space with two dimensions latitude and longitude, and we have time with two dimensions – calendar time, and the speed (instability) of the arrangement of the DNA base pairs. At any moment in linear calendar time there will be an arrangement of the DNA depending on the stability of the DNA. At one time there will be a clear set of ‘species’ and at another time a different set as the mix of characteristics continually change within and between the populations. ~ Lawrence Loucka
Haworthia mutica (groenewaldii) and its twisted leaves.
By M.B. Bayer
There is some argument about the status of this population of plants at Buffeljags. I have explained my opinion of it based on a species definition that I use. I also have reported on three other populations a short way away at Rotterdam. Furthermore I have discussed the flowers at length in a flower report. These are all available free on Haworthiaupdates.org. Gerhard Marx does not agree. The disagreement first has its roots in what constitutes a species and Marx stays with the standard view that characters are what define species. I opt for the view that species are systems related to geographic distribution and to all the elements that drive vegetation and change (evolution + equals change from some unknown initial condition). I think these patterns of change and difference are fractal i.e. detailed pattern repeating itself. Perhaps it would be more correct to say that the organization of pattern is according to a mathematical function which is non-linear. This means that the end product has many different outcomes. But this is complexity just of argument that none of us can deal with.
What comes into the geographic nature of species is also the nature of habitat. What happens is that we have a set of apparent species in Haworthia with a known distribution range. These species are primarily H. retusa and H. mirabilis. There is clear evidence that H. pygmaea and H. mutica emerge from a milieu of populations of those two and that H. floribunda is also involved. Buffeljags is geographically central to this arrangement and the habitat (wrongly described in the description of H. groenewaldii) is very unlike those where the named species generally occur. It is thus no surprise that the plants appear different. The Buffeljags population and its habitat also differ to a small degree from the west side of the river, but both are essentially geologically fairly recent river alluvial deposit.
Marx is insistent that the plants at Buffeljags are so different as to be a discrete species and I disagree. My disagreement is based on my experience of characters in plants. In Haworthia I think these are few and obscure. Thus it is almost impossible to delineate or circumscribe a species by characters and no one has succeeded in producing an identification key that can work. All the differences of opinion and argumentation about names come down to this issue of a species definition and the characters available to recognize them.
The essential points made for H. groenewaldii as a species is the shiny leaf surface and the flowering time. H. mutica does flower four months earlier – agreed. But H. marginata in the same close area also flowers similarly out of synchronicity with H. marginata elsewhere, as H. minima was also observed to do. In elaborating differences for H. groenewaldii, Marx offered the facts that the plants had fish-tail buds and that H. mutica did not. Very soon after he stated that he did not actually know what the case was in H. mutica.









Fig. 1 to 9, MBB7801 Haworthia mutica (groenewaldii), Buffeljags.
In addition he maintained that the leaves in H. groenewaldii were different as follows…”In terms of the angled newer leaves of H. groenewaldii, have a look at the plants again and you’ll see the young leaves are consistently twisted sideways. A spiraling effect. This never shows up in H. mutica”. I find this statement very odd because such a structural difference would come down to a difference on the level of genus or even family. So I looked at the plants I have in my possession and provide illustrations here to demonstrate no significant deviation in respect of twisting. I have even included a picture of a mature plant (fig. 10) of H. mirabilis to show the same “character”. The spiralling effect is universal in the aloids and is even visible in those species with distichous leaves. In the retusoids, where H. mutica belongs, the leaves have been said to be 5-farious. More usually it is possible to see them as trifarious. In young seedlings the leaves are bifarious as the very basic spiral effect comes into play.

I do not think this is a character one can use to distinguish a species at all. There are many cases where it is fairly possible to characterize populations by a wide range of so-called characters. My opinion is that generally in the many species (by my definition) of Haworthia this can virtually only be done at individual plant level. The Buffeljags and Rotterdam populations are simply the western counterparts of populations around Albertinia (eg H. mirabilis ‘splendens’, H. pygmaea ‘fusca’, H. pygmaea ‘esterhuizenii’) that emanate from the relationship of the prime species that I named above.





♦
Comprehension and significance
My writing has been described in all kinds of terms, hermetic and pretentious being two of the adjectives used. Recently Kris Tamayo also suggested that he had trouble understanding what I wrote or write. The fact is that writing and expressing yourself is difficult. But the first place is to be clear about what you want to say.
Writing is a means of communication and it really only should start when you are clear about what you think and what you want to say. Then it requires that the listener is clear about what one wants to hear and has the common cultural heritage that permits communication and understanding to occur?
In writing and talking about plants I personally get very frustrated by the technical problems of definition and knowledge that mess up communication completely. This is one of the obstacles in classification where there is no species definition and we do not actually know what species are. There are a lot of other obstacles. Recently someone wrote and implied that there were a lot of significant differences among Haworthia that could be used to arrive at a better classification (than any already available). The point I would make is that this person has his own idiosyncratic view of what significance means. This is not strange at all because a prominent scientist was once applying the statistical measure of standard deviation to two and three measured samples. That measure probably cannot be used until many more measurements are made. What is taken to be significant may be quite irrelevant to the actual question of whether there are more or less species. This is why amateurs and collectors should keep clear of classification. The professionals already have too many problems.
The characters we use to make identifications are important in that they may be of the yes and no kind i.e. present or absent, or they may be graded from vague to prominent. So it is very easy to go to one end of the scale and take only the prominent or what happens to strike your eye. This is exactly what happens. Unless followers and interested parties realize the impact this sort of decision making affects what they may want to know and understand, there can never be any harmony and peace in the classification process.
Look at these flowers and see what you can glean from them…



These just happen to be the only three flowers I have of a few plants of Haworthia herbacea from recent sampling. They are shown here in correct proportion to one another with the third being 18mm across at widest spread of the two upper outer petals. So we have two things we can call characters i.e. size and colour that we could say in respect of this simple sample, that they are significantly different. No matter how many times or how we measured these two things, this fact would stay the same. The plants happen to be from two populations and we can then ask if this is true for those populations. I did ask such a question of both H. herbacea and H. reticulata, and ended up by learning that I needed a sample of about 200 flowers to arrive at a statistically true answer at a probability of 95%. The thing is that I could go a little further south and sample another population and get a really pink flower with a spread of 25mm or more that would nearly double the spread of my measurements.
There are several incidentals here. One is the delineation of the mouth into the tube of the flower. Why is it so clear in the third picture? The second is that the first flower has not opened as flat across the face as the other two despite being at the same expected state in respect of time from opening. The third is that the name “subregularis” was used in this genre of flowers because the petals are so equally spread; perhaps less-so in the middle picture. Still a fourth curiosity is that in the southwestern species with the more extreme biarcuate bud with the fish-tail tip, is how the tips of the upper outer petals are “replicate” – i.e. the margins tend to fold together. In the Worcester/Robertson Karoo particularly H. herbacea and H. reticulata have the “regular” flower shape. But in H. mirabilis in this area, the upper outer petals may be held in a plane directly behind the inner outer petal and do not spread at all. There the bud tip is still fish-tail and the upper outer petal tips very replicate. ♦
Habitats





























































































MBB6694 Kanetvlei, Hex River Valley as a variant of Haworthia nortieri.
In Haworthia Update Vol. 9 dealing with H. maculata, I again draw attention to several populations that are problematic in an area very difficult to explore. The populations are MBB7865 at Keurkloof southeast of DeDoorns, EA1441 at the Hex River Pass, an unrecorded population south of Sandhills (about 3km east of Kanetvlei) reported by Ernst van Jaarsveld. In addition there are two populations apparently of H. arachnoidea at Tunnel Station east of Osplaas.
The illustrations that accompany this article are:
- MBB6694 Vreesniet, Kanetvlei.
- EA1441 Hex River Pass, east DeDoorns.
- MBB7865 ex E. Van Jaarsveld, Keurkloof, DeDoorns.
This population MBB6694 is at Kanetvlei southeast of Sandhills, and I originally ascribed it to H. arachnoidea but this is not correct. To this I can add unexamined populations reported by P.V. Bruyns deep in the Langeberg at Keerom Dam and another of Ted Oliver in the mountains north of Nuy. MBB6694 is only 200-300m north of MBB7994 also in sandstone. The sandstone strata are by no means all the same and one can even find shale bands in what is essentially a sandstone formation. H. maculata flowers late winter to early spring and these other populations in early summer.

I have not examined the flowers in any number and remain doubtful that this information will clarify any deductions that can be drawn from the vegetative characters or the geographic positions of the populations. It appears to me that consideration must be given to a relationship with H. nortieri that in my assessment occurs from the far northwest to as far east as Prince Albert, i.e. H. nortieri (devriesii). What I have seen of the flower, and shown now is that it definitely does not belong in the context of the Southern Cape species where the bud-tips tend to be “fish-tailed”. The inflorescence are slender and few flowered.
There is some criticism of my emphasis on geographical distribution as a key indicator of species. It was even said, as though I would have denied the fact, that I could not identify plants unless I knew where they came from. There is an incredible amount of both ignorance and the obtuse behind these statements. One critic feels that I have ignored detail and even ignored flower characters in the development of my opinions. This same critic claims a high degree of agreement with me (80%) based apparently on the view that so much of my classification is correct (meets with his approval), and presumably that the 20 percent that he contributes to the system makes it 100% correct. This is wholly wrong. This critic is simply unable to bridge the historical chasm between his introduction to, or knowledge of, Haworthia and mine. He simply does not recognise the shift in method and why I switched from a “character-based” approach to a phytogeographic one. It is really very simple in that the character based system as used from the very beginning of classification, has not produced a solution to the identification and classification of Haworthia. It all rests on a very weak and shaky definition of species determined by breeding barriers and consequently that morphological differences necessarily exist. My approach was to show that breeding barriers (at least in plants) could be integral to species and that morphological differences could equally be nothing but variation within species. Therefore I arrived at a definition of species from the view that they were phenomena spread in space and changing with time. This is just a fundamental of plant species as they constitute the vegetation and the floras of the world. Virtually the first question following “What is that?”, is “where does it come from?”.
Why 6694 is so interesting is precisely because it occurs in an area which is so poorly known and represented in our knowledge of Haworthia. The reality in the subgenus Haworthia is that there are several areas of great species richness and there is real pattern in the distribution of the various elements as they are listed in the 80% agreement area. The 20% disagreement zone concerns truly trivial opinion and unsubstantiated statements, and almost ignores the real reasons for disagreement at all. The real reasons are the realities that superficial morphological difference does not mean species and I have posted a vast amount of material that demonstrates that. I have shown repeatedly that differences in single characters in what can be rationally said to be the same species, can be greater than between different species.
In the case of 6694 I did not pick any particular character to identify the plants as H. arachnoidea from probably as far back as 1975 and I can find no earlier record of this location. I do recall a specimen in the Compton herbarium made by W.F.Barker that I mentally allied with H. arachnoidea but it is not cited as such in my revision. The identification was based on little else other than the spiny-ness of the plants and the fact that the nearest known locality for that species was a very new one of mine a few kilometres to the southwest. At the time, H. nortieri was barely known and this was from far to the northwest at Clanwilliam. Since then H. nortieri has become much better known although still some distance north of Ceres, and with its globose-flowered variants at Laingsburg and eastwards. H. arachnoidea remains remarkably unknown from the Cedarberg and Koue Bokkeveld mountains but does appear in the upper Hex River Valley. Here it is odd that the leaves tend to have the translucent dots that one would expect to characterise H. nortieri. But otherwise the leaf coloration is the much darker green that is associated with H. arachnoidea. A twist is that at Kunje, southeast of Citrusdal, H. nortieri does have very dark green leaves and there is no doubt that confusion with H. arachnoidea is inevitable. I have very little to offer in this regard because this degree and level of confusion or doubt is intrinsic to the genus anyway.
Why I now decide to relate this Kanetvlei population to H. nortieri is not to be construed as a decision now to call white what previously was clearly and definitely black. It is taking all my knowledge and experience to suggest simply that this is a better reflection of the situation where there is not enough hard data to determine identity at a higher level of certainty than about 50%. What I do suggest that there are many situations in Haworthia as the case with H. maculata and H. pubescens only recently exposed. In this case the two very different elements can be very confidently be said to be the same in respect of distribution and linking populations AND characters. Throughout, particularly the subgenus Haworthia, we have situations of continuity and spatial (geographic trend) and nearly all my many publications deal with exactly this reality. What it suggests is that my critic should come to consider if he has some other motive between punting points of difference that prevent him from finding points of agreement, besides those that rest coldly on logic.
1. MBB6694 Vreesniet, Kanetvlei.





























Flower profiles.










Flower faces.










Flower buds.



2. EA1441 Hex River Pass, east DeDoorns.


















3. MBB7865 ex E. Van Jaarsveld, Keurkloof, DeDoorns.








♦
Haworthia minima and pumila flowers
This H. pumila flower is apparently persistently regarded by botanists as actinomorphic (star-shaped, radially symmetrical) – as though zygomorphy (yoke shaped, bilateral, asymmetrical) in the aloids is an uncommon condition!
Radial symmetry means the flower can be divided into 3 or more identical sectors which are related to each other by rotation about the centre of the flower. Typically, each sector might contain one tepal or one petal and one sepal and so on. It may or may not be possible to divide the flower into symmetrical halves by the same number of longitudinal planes passing through the axis. Zygomorthic flowers can be divided by only a single plane into two mirror-image halves, much like a yoke or a person’s face.
If you see the way the inner upper petal overlaps BOTH the two lower inner petals, you recognise that there can not be actinomorphy in aloid flowers.
Haworthia pumila








Haworthia minima
















♦
MBB7989 Haworthia pumila, Lemoenpoort
Kobus drew my attention to a glabrous plant of Haworthia pumila while he was photographing this species at Lemoenpoort. The plants here have a missing chromosome and tend to have a purplish colour. I have seen smooth non-tubercled leaves elsewhere. But do check out that one plant – if you look carefully you can see the leaves are in 8 nearly vertical tiers. Proper botanists recently, for Taxon, described the arrangement of leaves like this in only two tiers (e.g. H. truncata) as “distichous insertion”. This is weird. Are the leaves in this plant of H. pumila “octichous”? Is H. viscosa “tristichous”. No, the leaves in the aloids are alternately and spirally inserted.


















Flower profiles



















Flower faces



















♦
Leaf arrangement in Aloe striatula
Herewith is an image of the leaf arrangement in Aloe striatula. I have numbered the leaves in inverse order to show that the leaves are as much distichious as trifarious i.e in two rows or three. The primary set is 1 through 10, the distichous set is 1, 3, 5, 7, 9 and 2, 4, 6, 8, 10. The trifarious set is 1, 4, 7, 10; 2, 5, 8 and 3, 6, 9. I have also added a picture of the leaf insertion – here it is entire and there is a leaf sheath around the stem – the actual point of insertion is at the pointer although the “veins” continue through that point to the stem itself. In this species the next leaf is inserted just below the opening of the previous sheath. Despite the leaves being alternate, they are spirally arranged. In Aloe broomii the leaf insertion is continuous and you can peel all the leaves off the stem in one piece. In most Haworthia the leaves are imbricate (overlapping alternately) but always in a spiral sequence. H. wittebergensis has leaves that have entire insertion and by memory the same is true for H. blackburniae and maybe for H. viscosa. ♦

Just what do we do with names for Haworthia?
Previously published in BCSJl (Cactus World) 30.4:211(2012)
M B Bayer
Taxonomy always provokes differing views, and Haworthia in particular has been subject to years of vacillation. The author has long been a campaigner against the haphazard proliferation of new names for every new, morphologically different population or variant. He questions the vagueness of a conventional species concept and pleads for a more reason-based, logical and sensible, communal approach to understanding and classifying Haworthia species. He hopes in this article to convey a message which is relevant to whatever genera of plants you grow. Photography by the author.
In Haworthia, professional botanists have struggled with and avoided the group because it is so infused with amateurs whose interest in the genus far outweighs any knowledge of botany. So classification of Haworthia has muddled on with scant regard for the discipline of botany as a science. Whether that has changed, is not for me to say. I have personally been gathering information on distribution and variation for over 50 years and have very seriously tried to keep that in the context of the science I was trained in and present it in a manner that botanists can follow and hopefully accept.
What has developed is that academic and professional botanists have been working with the tools of molecular biology and the results of five independent studies have all pointed towards the same conclusion, which is that the species of Haworthia are elusive, and the related genera are also not adequately distinguishable in the DNA data. The best solution they can offer is to merge the genera back into a single genus, namely Aloe. My personal reaction is that this is not a new idea and also Gordon Rowley pointed this out as far back as the 1970’s. I have also said that the subgenus Haworthia does not sit comfortably with the other two subgenera. Had I been a true taxonomist I would have implemented that by separating Haworthia into three separate genera and that is what I really would like to see. But the reality is that this does not solve the other problems that exist regarding Astroloba, Chortolirion, Poellnitzia, Chamaealoe, Leptaloe, Lomatophyllum, and the small Madagascan aloes. True botany alone can resolve the current and a new classification (still in manuscript form) has been proposed by a group of scientists that will really ‘rock the boat’ as far as collectors are concerned.
In Haworthiad (2012:4), I wrote about Haworthia mirabilis ‘submagnifica’. (Ed. note: The name in inverted commas, according to the author’s system indicates a variant name rather than a formal conventional variety, subspecies or form. It is his contention that there is no species definition and thus formal names have a large element of uncertainty. Another option is to drop the use of any rank denotation at all). It is one of the first populations linked to von Poellnitz’ H. magnifica long before so much was learned about distribution and variation. I used the prefix “sub” because this means “somewhat”, “almost”, “slightly”, “partially”, and possibly a few other words that mean… it is, but it is not. The population concerned is Komserante (Figs 1-2) and this particular population has acquired the name H. vernalis (Figs. 3-4). But I think we need to start from scratch and drop all the ‘baggage’ of the years. I personally have learned so much since I wrote my revision in 1996 that I know it is not possible to properly backtrack and retrace the passage forward by the use of Latin names. Interfertility is the basis of the system we use to identify and describe species and my field experience was already proving that this cannot possibly apply in the way in which Haworthia has been, or is to be, classified.


It is quite evident that H. retusa and H. mirabilis, both of which I accept in a very much broader context, do hybridise and there are populations that fit between. But first let me just explain that I regard H. retusa now to include H. turgida and all the variants of that species (nomenclatural priority obviated the use of the name turgida to cover the greater body of populations for this species). In the same way I regard H. maraisii, H. magnifica, and H. heidelbergensis and whatever variants were attached to those, as H. mirabilis. There are thus two species. My further observation is that H. pygmaea and H. mutica are segregates from the common gene pool of H. retusa and H. mirabilis. The Komserante population is the one in which that same gene pool is re-combining (Figs. 5-10). I am not in the least sure of all the intricacies but it seems to me that it is actually the group of populations that I recognize as H. retusa var. nigra that is pivotal in the relationship of all these species that I recognize. In this we discuss populations using names as prescribed by convention. This convention caters for chronology and authorship and not for evolutionary pathways. Both the names “retusa” and “turgida” precede the name “nigra”, but the populations that I now assign to H. retusa ‘nigra’ may better fit the concept of evolutionary origin.






The picture is complicated by the role of H. floribunda. This also hybridizes with both H. mirabilis and H. retusa as odd hybrids as well as at a population level. Just what are we to do? History has demonstrated all too well that a bevy of ill-assorted interested parties trying to impose a botanical classification is going to produce nothing but conflict and confusion. This has been going on now since the time that Smith, Von Poellnitz and Resende were simultaneously describing new species. How are we going to turn around and arrive at the understanding and stability of names that we seek?
In the past I have been extremely reluctant to make the following suggestion, and even now am a bit hesitant. What we need to do is turn to people who are employed to do this work. Herbaria and herbarium botanists are tasked and entrusted to classify and name plants. Perhaps it testifies to the complexity of the subject, or just its enormity, that these botanists have too often needed to defer to amateurs who have the interest, energy and enthusiasm and commitment to acquire field knowledge that a professional could never get the time or funds to do. The unfortunate part is that sometimes amateurs may not be able to relate their knowledge adequately to academic botany.
So what is the solution? It is that the community leaders assume the responsibility for the establishment of a system of classification that is meaningful to the community they serve. By community, I mean initially the botanists who should be providing us with scientifically sound classifications, then editors who are familiar with what botanists do, next are the Societies with their memberships, and also opinion formers in those groups. Finally included are the reviewers and commentators who lead opinion in one direction or another. It is surely not that difficult to sit down together to discuss and arrive at a set of guidelines by which a decision can be reached as to what (not whose) system to accept. A solution does not belong to anyone. Latin names are assumed to refer to an entity called a “species”. Botany has indeed been very lax and remiss in not providing a definition and this is the prime reason why amateurs have had so much freedom in generating Latin names for the most frivolous reasons. Botanists themselves have often not been far behind.
I think it is time to change all this and must excuse myself from any decision making body or process because I have a vested interest in respect of all the words I have written on the subject.
- Haworthia revisited. Umdaus Press, Hatfield. (1999)
- Plants in my collection 7: H. mirabilis ‘magnifica’. Haworthiad 26(1): 4-5 (2012)
♦
Nomenclator (2013)
The Haworthia nomenclator: a list of accepted species with some guidelines for infraspecific names
M.B. Bayer* and J.C. Manning**
*PO Box 960, Kuilsriver 7579, South Africa
**South African National Biodiversity Institute, P/Bag X7, Claremont 7735, South Africa
7 October 2012
(revised 1 February 2013)
With the impending revision of the IOS Succulent Lexicon and a growing awareness that the current classification of the genus Haworthia is unnatural, the time is ripe to propose a list of species names that I (MB) consider worthy of recognition. This list is essentially the same as that published recently in Haworthia Update 7(4): 30–40 (2012), in which we formally published numerous new combinations and new synonyms. There are two changes from that list implemented here. New information prompts us to recognise H. marxii as a species distinct from H. emelyae, and we transfer var. livida from H. pubescens to H. maculata also on the basis of new information.
My considered opinion is that there are at most about 60 species in Haworthia and that a better and more critical treatment would reduce this still more. This contrasts markedly with Breuer (2010), who lists 368 ‘accepted’ species and reports the even more startling claim that Hayashi recognises 550! Some of this increase is due to the tendency for these authors to treat my varietal names as full species but they have also described many new species de novo. I am unable to accept all of their proposed taxa in the context of my extensive field knowledge of the genus and my practical experience of patterns of variation among wild populations.
Species concepts in the genus have always been idiosyncratic, driven primarily by a propensity to recognize variation over similarity and fostered by a general ignorance of the extent of intra-population variability in the wild. The recent almost exponential proliferation of names has succeeded in surrounded the genus with a thicket of nomenclatural twigs that is almost impossible to penetrate and which obscures rather than illuminates any real understanding of the genus. The very appeal of the genus is its almost infinite capacity to vary, and it is a tragedy that they are no longer appreciated for their individuality but are instead pushed into or pulled out of taxonomic boxes almost willynilly. It is very difficult not to suspect the motivation behind some of this bedlam.
While we in no way wish to proscribe anybody from expressing their opinions, we issue an urgent plea for restraint. The nomenclatural flood that has all but submerged the genus will only be exacerbated by floating additional formal names and combinations on the turbulent waters. In fact, we go so far as to recommend a moratorium on the publication of any new taxa in the genus that are proposed without an extensive survey of relevant variation patterns in the wild. Scientific journals reduce the chances that inadequately researched papers will see the light of day by sending all submissions to competent referees for comment and evaluation. Popular journals that transgress into the scientific arena by accepting descriptions of new taxa should do the same and the responsibility for this lies firmly with the editors.
Every scientific name that enters currency has to be accounted for by botanists in their lists and publications. To remove them from circulation requires a separate, formal act for each and every name. Dealing with the many dubious names in Haworthia is taxing task that can be ill afforded. Numerous invalidly published or illegitimate names have also entered circulation through non-adherence to the International Code of Botanical Nomenclature, which aims to regulate scientific names. This adds to the confusion and to the work.
What in fact is the primary purpose of these scientific names? Among botanical circles, species names carry a great deal of weight. In most instances they imply that individuals that share a name also share a common ancestry and can interbreed to produce viable seeds. Species with different names will not. Is this philosophy applied in Haworthia? The process of recognising species in botanical circles is generally top-down, with genera being first split into component species and the species then into subspecies or varieties acording to the particular patterns of variation shown by the constituent populations. This does not seem to apply in Haworthia. Here the process is largely bottom-up, with individuals or populations being given names and then shuffled into or among species. This is patently absurd.
In general terms, botanical names largely reflect similarities whereas horticultural names highlight differences. The two systems are thus not always congruent and little is to be served by confusing them. We therefore recommend that growers exploit the advantages of an informal system of nomeclature that will give them unlimited flexibility without clogging up the formal nomenclature any further. We also urge the Editors of popular journals to encourage the use of this system among their contributers. The great advantage of such an informal and non-ranked system is that it removes the hierarchical constraints that are inherent in the Linnaean system and that have already created problems with the placement of varieties and forms of Haworthia.
It could not be easier to implement and use: instead of worrying about the taxonomic level at which the variant is to be recognized (is it a subspecies, or a variety, or a form) the name appears in inverted commas without any indication of its rank. Such informal names can also be moved between species without any formal changes that are otherwise necessary. They are likely to be more descriptive and meaningful than a bare Latinised trivial epithet. As an example, should Mr Smith consider that the form of H. mirabilis var. sublineata from south of Bredasdorp with paler, attenuate leaves deserves special recognition, then he might usefully refer to those plants under the informal name H. mirabilis ‘Bredasdorp Pale’ Smith 2015. A typical illustration should be designated to fix the application of the name. Transferring of the variant to another species should follow the Zoological Code in carrying over the first author only (now in brackets) and not the transferring author as well. Thus: H. mirabilis ‘Bredasdorp Pale’ (Smith 2015). We are most grateful to Harry Mays, Editor of the Haworthia Update for his assistance and advice on this aspect.
THE LIST: All taxa that we accept are indicated in bold.Taxa that are not accepted at any rank are included as synonyms. Where possible we formally synonomise such names that have not yet been formally synonomised but many others (indicated with an asterisk *) do not appear in the International Plant Names Index (www.ipni.org) as of 20 January 2012 and are thus evidently unpublished or manuscript name. The publication of such names in the literature is irresponsible. Names that are separated by a full stop (.) are based on different types (heterotypic or taxonomic synonyms) whereas those separated by a colon (:) are based on the same type (homotypic or nomenclatural synonyms).
Haworthia Duval Pl. Succ. Horto. Alenc.: 7pp (1809). Baker in Jl. S. Afr. Bot. 18: 197(1880). Baker in Fl. cap. 6: 332 (1896). Berger in Das. Pfl. 4: 74 (1908). Bayer, New Haworthia Handb. (1982). Scott, The genus Haworthia (1985). Type species: H. arachnoidea (L.) Duval. [Typification largely by Breuer & Metzing in Taxon (1996)].
= Catevala Medik. (1786). Apicra Willd. (1811).
± 60 species, very variable and complex. The taxonomy of the genus is still unresolved. Species concepts used here largely follow Bayer’s (1976, 1982, 1997) treatments and are based on geographical distribution and co-occurrence.
NOTE: Recent phylogenetic analyses of nuclear and plastid DNA sequence data supports the view that the three subgenera comprise quite distinct lineages not immediately related to one another. They are thus as distinct as some of the other smaller genera and should thus logically be treated as separate genera. A more useful option is probably to include all of the alooid genera within Aloe.
Key to the subgenera
1. Flowers triangular or rounded-triangular at base; tube obclavate-curved; outer tepals free; style upcurved; seeds irregularly angled . . . subg. Haworthia
2. Flowers hexangular at base, gradually narrowing to junction with pedicel (substipitate); tube obcapitate-curved; outer tepals partly fused to inner; style straight; seeds irregularly angled . . . subg. Hexangulares
3. Flowers rounded at base and abruptly joined to pedicel (non-stipitate); petals partly fused; tube obcapitate-straight; style straight; seeds flattish . . . subg. Robustipedunculatae
I. Subgenus Haworthia. Type species: as for genus. ± 41 spp.
H. angustifolia Haw. in Philos. Mag. J. 66: 283 (1825). Neotype, designated by Breuer & Metzing (1997): Grahamstown to Alicedale, Bruyns 1653 (NBG).
= Aloe stenophylla Schult. & Schult.f. (1829). H. albanensis Shonl. (1912). H. angustifolia var. grandis Smith (1943).
H. angustifolia var. angustifolia
H. angustifolia var. altissima Bayer in Haw. Revis.: 26 (1999): H. altissima (Bayer) M.Hayashi in Haworthia Study 3: 13 (2000). Type: Riebeek East to Carlisle Bridge, Smith 5220 (NBG).
H. angustifolia var. baylissii (Scott) Bayer in Haw. Revis.: 27 (1999): H. baylissii Scott (1968). Type: Oudekraal, Zuurberg, Bayliss sub Scott 796 (PRE).
H. angustifolia var. paucifolia Smith in Jl. S. Afr. Bot. 14: 48 (1948): H. paucifolia (Smith) M.Hayashi in Haworthia Study 22: 11 (2010). Type: Frazers Camp, Smith 6819 (NBG).
H. arachnoidea (L.) Duval in Pl. Succ. Hort. Alenc.: 7 (1809): Aloe pumila var. arachnoidea L. (1753): Catevala arachnoidea (L.) Medik. (1786): Apicra arachnoidea (L.) Willd. (1811). Lectotype, designated by Scott (1977): Commelin, Praeludia Bot.: t. 27 (1703). Epitype, designated by Breuer & Metzing (1997): Buitenstekloof, Langvlei, Bayer 153 (NBG).
= H. arachnoidea var. minor Haw. (1819).
H. arachnoidea var. arachnoidea
= H. joubertii M.Hayashi in Haworthia Study 14: 16 (2005), nom. inval. H. laxa M.Hayashi in Haworthia Study 14: 14 (2005), nom. inval. H. limbata M.Hayashi in Haworthia Study 14: 16 (2005), nom. inval. *H. isomorpha *H. gilva
H. arachnoidea var. aranea (Berger) Bayer in Haw. Revis.: 30 (1999): H. bolusii var. aranea Berger (1908): H. aranea (Berger) Bayer (1976). Lectotype: Engler, Pflanzenr. 33: 114, f. 39 A–E (1908). Epitype, designated by Breuer & Metzing (1997): Robinson Pass, Moeras River Drift, Bolus 12372 (BOL).
H. arachnoidea var. namaquensis Bayer in Haw. Revis.: 31 (1999): H. namaquensis (Bayer) Breuer in Gen. Haworthia 1: 7 (2010). Type: Karrachabpoort, Richtersveld, Bayer 1674 (NBG).
H. arachnoidea var. nigricans (Haw.) Bayer in Haw. Revis.: 32 (1999): H. setata var. nigricans Haw. (1821). Neotype, designated by Bayer (1997): SW Vanwyksdorp, Bayer 2419 (NBG).
= H. helmiae V.Poelln. (1937): H. unicolor var. helmiae (V.Poelln.) Bayer (1976). H. venteri V.Poelln. (1939): H. unicolor var. venteri (V.Poelln.) Bayer (1976). H. scottii Breuer in Avonia 21: 55 (2003). H. nigrata M.Hayashi in Haworthia Study 15: 14 (2006). *H. apata *H. regens *H. formosa *H. kuromisa
H. arachnoidea var. scabrispina Bayer in Haw. Revis.: 34 (1999): H. scabrispina (Bayer) Breuer in Gen. Haworthia 1: 8 (2010). Type: Baviaans, Bayer 2105 (NBG) *H. matjiesta
H. arachnoidea var. setata (Haw.) Bayer in Haw. Revis.: 34 (1999): H. setata Haw. (1819). Iconotype: artist unknown, specimen received from Dr Mackrill ex Cape (K).
= H. setata var. media Haw. (1821). H. setata var. major Haw. (1821). Aloe setosa Schult. & Schult.f. (1829). H. gigas V.Poelln. (1932): H. setata var. gigas (V.Poelln.) V.Poelln. (1938). H. minima var. major V. Poelln. (1938): H. tenera var. major (V.Poelln.) Uitew. (1948). H. pectinis M.Hayashi in Haworthia Study 10: 13 (2003). H. tretyrensis Breuer in Avonia 21: 58 (2003). H. candida M.Hayashi in Haworthia Study 16: 16 (2006). H. cangoensis M.Hayashi in Haworthia Study 14: 13 (2005), nom. inval. H. angiras M.Hayashi in Haworthia Study 14: 13 (2005), nom. inval. H. kogmansensis M.Hayashi in Haworthia Study 14: 14 (2005), nom. inval.
H. aristata Haw. in Suppl. Pl. Succ.: 51 (1819). Iconotype: (K). Epitype: Deadman’s Gulch (Soutkloof), Smith 3550 (NBG).
= H. denticulata Haw. (1821). H. lapis Breuer & M.Hayashi in Alsterworthia Int. Special Issue 7: 6 (2004). H. rava M.Hayashi in Haworthia Study 14: 11 (2005), nom. inval.
H. bayeri Hammer & Venter in Cact. Succ. J (US) 69: 75 (1997). Type: S Uniondale, Stayner in KG164/69 (NBG).
= H. hayashii M.Hayashi in Haworthia Study 7: 14 (2002). H. laeta M.Hayashi in Haworthia Study 12: 13 (2004). H. indigoa M.Hayashi in Haworthia Study 12: 13 (2004). H. truterorum Breuer & Marx in Aloe 48: 54 (2011).
H. blackburniae Barker in J. S. Afr. Bot. 3:93 (1937). Type: Calitzdorp, Reynolds 1842 (NBG).
H. blackburniae var. blackburniae
H. blackburniae var. graminifolia (Smith) Bayer in Haw. Revis.: 42 (1999): H. graminifolia Smith (1942). Type: Schoemanspoort, M. Courtenay‑Latimer in Smith 5222 (NBG).
H. blackburniae var. derustensis Bayer in Haw. Revis.: 41 (1997): H. derustensis (Bayer) M.Hayashi in Haworthia Study 3: 13 (2000). Type: W. De Rust, Vlok sub Venter 93/24 (NBG).
H. bolusii Baker in J. Linn. Soc. Bot.:215 (1880). Type: Graaff-Reinet, Bolus 158 (K).
= H. odetteae Breuer in Avonia 21: 51 (2003). H. odyssei M.Hayashi in Haworthia Study 14: 11 (2005), nom. inval. H. capillaris M.Hayashi in Haworthia Study 16: 16 (2006).
H. bolusii var. bolusii
H. bolusii var. blackbeardiana (V. Poelln.) Bayer in Haw. Hand.: 31 (1976): H. blackbeardiana V.Poelln. (1932). Lectotype, designated by Breuer & Metzing (1997): ex cult. V.Poelln. 1932 (B).
= H. blackbeardiana var. major V.Poelln. (1937). H. inermis V.Poelln. (1932): H. altilinea var. inermis (V.Poelln.) V.Poelln. (1937): H. altilinea var. limpida f. inermis (V.Poelln.) V.Poelln. (1940). H. batteniae Scott (1979). H. calaensis Breuer in Alsterworthia Int. Special Issue 7: 5 (2004). H. specksii Breuer in Alsterworthia Int. Special Issue 7: 8 (2004).*H. hogsia *H. speciosa *H. malvina
H. bolusii var. pringlei (Scott) Bayer in Haworthiad 16: 62 (2002). H. decipiens var. pringlei (Scott) Bayer in Haw. Revis.: 67 (1999): H. pringlei Scott (Bradleya 12:103,1994). Type: Adelaide district, Scott s.n. PRE 8970 (PRE). H. hisui M.Hayashi in Haworthia Study 12: 10 (2004). H. lazulis M.Hayashi in Haworthia Study 14: 11 (2005), nom. inval. H. aquamarina M.Hayashi in Haworthia Study 10: 13 (2003). H. hastata M.Hayashi in Haworthia Study 12: 9 (2004).
H. chloracantha Haw. in Revis.:57 (1821): Aloe chlorocantha (Haw.) Scult. & Schult.f. (1829). Neotype, designated by Breuer & Metzing (1997): N Herbertsdale, Bayer KG411/75 (NBG).
H. chloracantha var. chloracantha
H. chloracantha var. denticulifera (V.Poelln.) Bayer in Haw. Hand.: 112 (1976): H. angustifolia var. denticulifera V.Poelln. (1937): H. denticulifera (V.Poelln.) M.Hayashi in Haworthia Study 3: 13 (2000). Type (icono.): (B).
= H. angustifolia var. lilliputana Uitew. (1953).
H. chloracantha var. subglauca V.Poelln. in Kakteenkunde 9:135 (1937): H. subglauca (V.Poelln.) M.Hayshi in Haworthia Study 3: 13 (2000). Neotype, designated by Breuer & Metzing (1997): Great Brak, Hurling & Neil (BOL).
H. cooperi Baker in Refug. Bot. 4: 233 (1871). Type: Cape, Cooper (K).
= H. vittata Baker (1871).
H. cooperi var. cooperi
= H. pallens Breuer & M.Hayashi in Alsterworthia Int. Special Issue 7: 7 (2004). *H. turcosa *H. elegans *H. foeda *H. yocans
H. cooperi var. dielsiana (V.Poelln.) Bayer in Haw. Revis.: 51 (1999): H. dielsiana V.Poelln. (1930): H. pilifera var. dielsiana (V.Poelln.) V.Poelln. (1940): H. obtusa var. dielsiana (V.Poelln.) Uitew. (1948). Neotype, designated by Bayer (1999): Sheldon, A.J. van der Merwe in Smith 1140 (NBG).
= H. joeyae Scott (1975).
H. cooperi var. doldii Bayer in Haworthiad 16: 65 (2002): H. doldii (Bayer) M.Hayashi in Haworthia Study 14: 11 (2005). Type: Chalumna, Dold 3961 (GRA).
H. cooperi var. gordoniana (V.Poelln.) Bayer in Haw. Revis.: 52 (1999): H. gordoniana V.Poelln. (1937): H. pilifera var. gordoniana (V.Poelln.) V.Poelln. (1938): H. obtusa var. gordoniana (V.Poelln.) Uitew. (1948). Neotype, designated by Bayer (1999): Patensie, Smith 3028 (NBG).
= H. harryi M.Hayashi in Haworthia Study 12: 9 (2004). H. jeffreis M.Hayashi in Haworthia Study 12: 10 (2004). H. pusilla M.Hayashi in Haworthia Study 12: 10 (2004). H. ligulata M.Hayashi in Haworthia Study 12: 6 (2004). H. venetia M.Hayashi in Haworthia Study 12: 6 (2004). *H. brandea *H. cineraria *H. compressa *H. gelatina *H. ionandra *H. neritica *H. silvicola *H. tomentosa
H. cooperi var. gracilis (V.Poelln.) Bayer in Haworthiad 16: 64 (2002): H. gracilis V.Poelln. (1929). Neotype, designated by Bayer (1999): Hellspoort, Britten (PRE).
= H. caerulea M.Hayashi & Breuer in Haworthia Study 12: 7 (2004).
H. cooperi var. isabellae (V.Poelln.) Bayer in Haworthiad 16: 62 (2002): H. gracilis var. isabellae (V.Poelln.) Bayer in Haw. Revis.: 77 (1999): H. isabellae V.Poelln. (1938). Neotype, designated by Bayer: Humansdorp, Gamtoos bridge, H. Hall sub NBG 68799 (NBG).
= H. azurea M.Hayashi in Haworthia Study 9: 12 (2003). H. arabesqua M.Hayashi in Haworthia Study 12: 7 (2004). H. bella M.Hayashi in Haworthia Study 12: 8 (2004). H. florens M.Hayashi in Haworthia Study 12: 11 (2004). H. pilosa M.Hayashi in Haworthia Study 12: 7 (2004). H. bathylis M.Hayashi in Haworthia Study 15: 116 (2006). H. lachnosa M.Hayashi in Haworthia Study 16: 16 (2006). H. ciliata M.Hayashi in Haworthia Study 14: 11(2005), nom. inval. *H. kromia. *H. patriae *H. cuprina *H. dasylis
H. cooperi var. leightonii (Smith) Bayer in Haw. Hand.: 128 (1976): H. leightonii Smith (1950). Type: Kayser’s Beach, Smith 6938 (NBG).
= Haworthia leightonii var. davidii Breuer in Avonia 21: 49 (2003).: Haworthia davidii (Breuer) M.Hayashi & Breuer (2005). Type: SW East London, Breuer 6970 (TUAT). *H. sabita?
H. cooperi var. minima (Bayer) Bayer (2012): H. minima Baker (1880) hom. illegit. non (Aiton) Haw. (1812): H. gracilis var. minima Bayer [as (Baker) Bayer] (1999). Iconotype: (K).
= H. tenera V.Poelln. (1932): H. translucens subsp. tenera (V.Poelln.) Bayer (1976): H. gracilis var. tenera (V.Poell.) Bayer (1999): H. cooperi var. tenera (V.Poelln.) Bayer (2002). H. cummingii Breuer & M.Hayashi in Haworthia Study 10: 4 (2003).
H. cooperi var. picturata (Bayer) Bayer in Haworthiad 16: 65 (2002): H. gracilis var. picturata Bayer (1999): H. picturata M.Hayashi (2000). Type: Enon, Thode 21507 (NBG).
= H. oculata M.Hayashi in Haworthia Study 12: 10 (2004). *H. florida *H. imperialis *H. kubusie
H. cooperi var. pilifera (Baker) Bayer in Haw. Revis.: 54 (1999): H. pilifera Baker (1871): H. obtusa var. pilifera (Baker) Uitew. (1948). Iconotype: Refug. Bot.: 234 (1871).
= H. stayneri V.Poelln. (1937): H. pilifera var. stayneri (V.Poelln.) V.Poelln. (1938): H. obtusa var. stayneri (V.Poelln.) Uitew. (1948). H. stayneri var. salina V.Poelln. (1937): H. pilifera var. salina (V.Poelln.) V.Poelln. (1938): H. obtusa var. salina (V.Poelln.) Uitew. (1948): H. salina (V.Poelln.) M.Hayashi (2010). H. pilifera var. dielsiana f. acuminata V.Poelln. (1940): H. obtusa var. dielsiana f. acuminata (V.Poelln.) Uitew. (1948). H. luri M.Hayashi in Haworthia Study 14:11 (2005), nom. inval. *H. sabrina
H. cooperi var. truncata (Jacobs.) Bayer in Haw. Rev.: 55 (1999): H. obtusa var. pilifera f. truncata Jacobs in Nat. Cact. Succ. J. 10: 81 (1955): H. ikra Breuer (2010). Neotype, designated by Bayet (1999): Runlets, Mgwali, Smith 5295 (NBG).
H. cooperi var. venusta (Scott) Bayer in Haw. Revis. (1999): H. venusta Scott in Bradleya 14:87 (1996). Type: NE Alexandria, Britten 781 (GRA).
H. cooperi var. viridis (Bayer) Bayer in Haworthiad 16: 65 (2002): H. gracilis var. viridis Bayer (1999). Type: Perdepoort, Smith 6867 (NBG).
= H. hamata M.Hayashi in Haworthia Study 10: 12 (2003). H. emeralda M.Hayashi in Haworthia Study 12: 11 (2004). H. subhamata M.Hayashi in Haworthia Study 12: 11 (2004). H. teres M.Hayashi in Haworthia Study 12: 7 (2004). *H. swannea
H. cymbiformis (Haw.) Duv. in Pl. Succ. Hort. Elenc.:7 (1809): Aloe cymbiformis Haw. (1804): H. concava Haw., nom. illegit. superfl. (1821). Neotype, designated by Breuer & Metzing (1997): Port Elizabeth, Walmer, Smith 2844 (NBG).
= H. planifolia Haw. (1825): H. cymbiformis var. planifolia (Haw.) Baker (1880): Aloe planifolia (Haw.) Salm-Dyck (1840). H. cymbiformis var. angustata V.Poelln. (1938): H. angustata (V.Poelln.) Breuer in Gen. Haw. 1: 7 (2010). H. cymbiformis var. angustata f. subarmata V.Poelln. (1938). H. cymbiformis var. compacta Triebn. (1938): H. compacta (Triebn.) Breuer in Gen. Haw. 1: 7 (2010). H. planifolia var. exulata V.Poelln. (1938). H. planifolia var. planifolia f. agavoides Triebn. & V.Poelln. (1938), et f. olivacea Triebn. & V.Poelln. (1938), et f. robusta Triebn. & V.Poelln. (1938), et var. incrassata V.Poell. (1938), et var. sublaevis V.Poelln. (1938), et var. longifolia Triebn. et V.Poelln. (1938), et var. longifolia f. calochlora Triebn. et V.Poelln. (1938). H. planifolia var. poellnitziana Resende (1943). H. lepida Smith (1944). *H. cana *H. ingens *H. plena *H. rosea
H. cymbiformis var. cymbiformis
H. cymbiformis var. incurvula (V.Poelln.) Bayer in Haw. Hand.: 124 (1976): H. incurvula V.Poelln. (1932). Neotype, designated by Breuer & Metzing (1997): Pluto’s Vale, Britten s.n. BOL71307 (BOL).
H. cymbiformis var. obtusa (Haw.) Baker in J. Linn. Soc. Bot. 18: 209 (1880). H. obtusa Haw. in Phil.Mag. 46: 282 (1825). Iconotype: (K).
= H. umbraticola V.Poelln. (1937): H. cymbiformis var. umbraticola (V.Poelln.) Bayer (1976). H. hilliana V.Poelln. (1937): H. umbraticola var. hilliana V.Poelln. (1938). H. obtusa var. pilifera f. truncata Jacobs. (1960). *H. blinkia
H. cymbiformis var. ramosa (Smith) Bayer in Haw. Revis.: 60 (1999): H. ramosa Smith (1940): H. cymbiformis f. ramosa (Smith) Bayer (1976). Type: Wooldridge, Smith 3168 (NBG).
H. cymbiformis var. setulifera (V.Poelln.) Bayer in Haw. Revis.: 62 (1999): H. planifolia var. setulifera V.Poelln. (1938): H. setulifera (V.Poelln.) Breuer (2010). Neotype, designated by Bayer (1999): Kwelegha Bridge, Smith 5257 (NBG). H. cymbiformis var. obesa V.Poelln. (1938): H. obesa (V.Poell.) Breuer. *H. sarcoidea
H. decipiens V.Poelln. in Repert. Spec. Nov. Regni. Veg. 28: 103 (1930). Neotype, designated by Breuer & Metzing (1997)): Prince Albert, Kleinsleutelfontein, Bayer 5157 (NBG).
= H. exilis M.Hayashi in Haworthia Study 10: 12 (2003). H. incrassa M.Hayashi in Haworthia Study 14: 12 (2005), nom. inval. *H. tooris
H. decipiens var. decipiens
H. decipiens var. cyanea Bayer in Haw. Revis.: 65 (1999): H. cyanea (Bayer) Hayashi (2000). Type: Fairview, W Jansenville, Bayer 4180 (NBG).
= H. amethysta M.Hayashi in Haworthia Study 10: 1 (2003). H. succinea M.Hayashi in Haworthia Study 10: 14 (2003). H. ianthina M.Hayashi in Haworthia Study 14: 12 (2005), nom. inval.*H. virginea
H. decipiens var. minor Bayer in Haw. Revis.: 66 (1999). Type: Kleinpoort, Smith 3588 (NBG). *H. tenmari
H. decipiens var. virella Bayer in Haworthiad 16: 63 (2002). Type: Ebenezer, Bayer 2070 (NBG).
= H. crinita M.Hayashi in Haworthia Study 10: 13 (2003). H. eminens M.Hayashi in Haworthia Study 10: 13 (2003). H. floccosa M.Hayashi in Haworthia Study 10: 13 (2003). H. kemari M.Hayashi in Haworthia Study 9: 11 (2003). H. fluffa M.Hayashi in Haworthia Study 12: 9 (2004). H. jansenvillensis Breuer in Alsterworthia Inst. 4: 16 (2004). H. pellucida M.Hayashi in Haworthia Study 14: 12 (2005), nom. inval. *H. delicata *H. ionides *H. lanceata *H. stewarta
H. decipiens var. xiphiophylla (Baker) Bayer in Haworthiad 16: 63 (2002). H. arachnoidea var. xiphiophylla (Baker) Bayer in Haw. Revis.: 36 (1999). Haworthia xiphiophylla Baker, Curtis’ Bot.Mag.: t. 7505 (1896). H. setata var. xiphiophylla (Baker) V. Poelln. (1938). Type: Uitenhage, Howlett (K).
= H. longiaristata V. Poelln. (1937). H. flavida M.Hayashi in Haworthia Study 10: 13 (2003). *H. kammaensis
H. emelyae V.Poelln. in Repert. Spec. Nov. Regni. Veg. 42: 271 (1937). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] (B).
= H. blackburniae V.Poelln. (1937). H. correcta V.Poelln. (1938). H. picta V.Poelln. (1938). H. picta var. janvlokii Breuer in Avonia 21: 52 (2003). H. janvlokii (Breuer) Breuer (2010). H. breueri M.Hayashi in Haworthia Study 7: 14 (2002). H. picta var. tricolor Breuer in Avonia 21: 53 (2003).: H. tricolor ( Breuer ) M.Hayashi (2010).
H. emelyae var. emelyae
H. emelyae var. comptoniana (Smith) Hammer and Venter in Cact. Succ. J. (US) 69: 77 (1997): H. comptoniana Smith (1945). Type: Georgida, Malherbe sub Smith 3433 (NBG).
H. emelyae var. major (Smith) Bayer in Haw. Revis.: 70 (1999): H. schuldtiana var. major Smith (1946): H. maraisii var. major (Smith) Bayer (1976): H. magnifica var. major (Smith) Bayer (1977). Type: Garcia’s Pass, Smith 5370 (NBG).
= H. wimii M.Hayashi in Haworthia Study 3: 13 (2000).
H. emelyae var. multifolia Bayer in Nat. Cact. Succ. J. 34:31 (1979): H. multifolia (Bayer) M.Hayashi (2000). Type: Riversdale, Springfontein, Bayer 1558 (NBG).
H. floribunda V.Poelln. in Repert. Spec. Nov. Regni Veg. 40: 149 (1936). Neotype, designated by Bayer (1982): [unpublished image] (B). Epitype, designated by Breuer & Metzing (1997): Blackdown, NE Heidelberg, Bayer 158 (NBG).*H. henda
H. floribunda var. floribunda
H. floribunda var. dentata Bayer in Haw. Revis.: 73 (1999): H. dentata (Bayer) M.Hayashi (2000), hom. illegit. non H.Jacobsen (1935). Type: W Riversdale, Dekenah 90 sub Smith 5502 (NBG).
H. floribunda var. major Bayer in Haw. Revis.: 74 (1999). Type: S Swellendam, De Kok (NBG).
= H. kondoi M.Hayashi in Haworthia Study 3: 13 (2000).
H. herbacea (Mill.) Stearn in Cactus J. 7: 40 (1938): Aloe herbacea Mill. (1768). Lectotype, designated by Bayer (1972): Illustration in Boerhaave, Index Alter Hort. Lugd.-Bat. 2: 130: t. 131 (1720). Epitype, designated by Breuer & Metzing (1997): N Ribbokkop, Bayer 161 (NBG).
= Aloe atrovirens DC. (1799): H. atrovirens (DC.) Haw. (1821). H. pumila (Willd.) Duval (1809). Aloe translucens Haw. (1804): H. translucens (Haw.) Haw. (1819): Aloe arachnoidea var. translucens (Haw.) Ker-G. (1811). H. pellucens Haw. (1812). H. pallida Haw. (1821). H. paynei V.Poelln. in Feddes Repert. 41: 206 (1937).: H. herbacea var. paynei (V.Poelln.) Bayer (1999). H. aegrota V.Poelln. (1939). H. submaculata V.Poelln. (1939). H. luteorosea Uitew. (1939).
H. herbacea var. herbacea
H. herbacea var. flaccida Bayer in Haw. Revis.: 86 (1999): H. flaccida (Bayer) Breuer (2010). Type: Worcester, Rooiberg, Bruyns (NBG).
H. herbacea var. lupula Bayer in Haw. Revis.: 86 (1999): H. lupula (Bayer) M.Hayashi (2000). Type: Villiersdorp, Boscheveld Mt., Wolfkloof, Esterhuysen (NBG).
H. lockwoodii Archibald in Fl. Pl. Africa 20: f. 792 (1940). Type: near Laingsburg, Lockwood-Hill 215 (GRA).
H. maculata (V.Poelln.) Bayer in Haw. Hand.: 130 (1976): H. schuldtiana var. maculata V.Poelln. (1940). Lectotype, designated by Breuer & Metzing (1997): Worcester, Venter 6 (BOL).
H. maculata var. maculata. *H. audens
H. maculata var. livida (Bayer) Bayer, comb. nov.: H. pubescens var. livida Bayer in Haw. Revis.: 134 (1999). Type: Worcester, Lemoenpoort, Bayer 1128 (NBG). *H. livida
H. marumiana Uitew. in Cact.Vetp. 6: 33 (1940). Type: Cape, Ladismith, ex hort. Stellenbosch sub 6610 (AMD).
= H. borealis M.Hayashi in Haworthia Study 15: 14 (2006). H. marmorata M.Hayashi in Haworthia Study 15: 14 (2006). H. tarkasia M.Hayashi in Haworthia Study 15: 14 (2006). *H. euchlora
H. marumiana var. marumiana
H. marumiana var. archeri (W.F.Barker ex Bayer) Bayer in Haw. Revis.: 104 (1999): H. archeri W.F.Barker ex Bayer (1981). Type: Whitehill, Archer s.n. NBG 68145 (NBG). *H. chibita *H. frazeri *H. nudata
H. marumiana var. batesiana (Uitew.) Bayer in Haw. Revis.: 105 (1999): H. batesiana Uitew. (1948). Type: Graaff-Reinet, Ferguson (AMD).
H. marumiana var. dimorpha (Bayer) Bayer in Haw. Revis.: 106 (1999): H. archeri var. dimorpha Bayer (1981): H. dimorpha (Bayer) M.Hayshi (2000). Type: Constable Station, W Laingsburg, Hall sub Smith 7418 (NBG).
H. marumiana var. reddii (Scott) Bayer (2012): H. cymbiformis var. reddii (Scott) Bayer (1999): H. reddii Scott in Cact. Succ. J. (US) 66:182 (1994). Type: Cathcart, Waterdown Dam, Scott 8968 (PRE). *H. boloensis *H. fatreddii
H. marumiana var. viridis Bayer in Haw. Revis.: 107 (1999). Type: S Prince Albert, Bayer 3620 (NBG). *H. viridis
H. marxii Gildenh. in Aloe 44: 4 (2007). Type: Western Cape, Rooinek Pass, Marx 605 (GRA, holo.).
H. mirabilis (Haw.) Haw. in Syn. Pl. Succ.: 95 (1812): Aloe mirabilis Haw. (1804). Neotype, designated by Bayer (1977): Illustration in Curtis’ Bot. Mag.: t. 1354 (1811). Epitype, designated by Breuer & Metzing (1997): Skuitsberg, between Caledon and Greyton, Bayer 2453 (NBG).
H. mirabilis var. mirabilis
H. mirabilis var. atrofusca (Smith) Bayer (2012): H. atrofusca Smith in Jl. S. Afr. Bot. 14:41 (1948): H. magnifica var. atrofusca (Smith) Bayer (1977). Type: Dekenah 225 sub Smith 6169 (NBG).
= H. enigma M.Hayashi in Haworthia Study 7: 14 (2002).
H. mirabilis var. badia (V.Poelln.) Bayer in Haw. Revis.: 109 (1999): H. badia V.Poelln. (1938): Haworthia mirabilis subsp. badia (V.Poelln.) Bayer (1976). Lectotype, designated by Bayer (1977): Illustration in Kakteenk. en Kakteenfr.: 76 (1938).
H. mirabilis var. beukmannii (V.Poelln.) Bayer in Haw. Revis.: 110 (1999): H. emelyae var. beukmannii V.Poelln. (1940): H. beukmannii (V.Poelln.) M.Hayashi (2000). Type: [unpublished image] (B). Epitype, designated by Bayer (1999): Caledon, Skuitsberg, Smith 3969 (NBG).
H. mirabilis var. consanguinea Bayer in Haw. Revis.: 111 (1999): H. consanguinea (Bayer) M.Hayashi (2000). Type: Die Galg, Bayer (NBG).
H. mirabilis var. heidelbergensis (Smith) Bayer (2012): H. heidelbergensis Smith in Jl. S. Afr. Bot. 14:42 (1948). Type: W Heidelberg, J. Dekenah 230 in Smith 6566 (NBG). *H. obscura?
H. mirabilis var. magnifica (V.Poelln.) Bayer (2012): Haworthia magnifica V.Poelln. in Repert. Spec. Nov. Regni Veg. 33: 240 (1933): H. maraisii var. magnifica (V.Poelln.) Bayer : 131 (1976). Lectotype, designated by Breuer & Metzing (1997): Riversdale, Ferguson (BOL).*H. vernalis
H. mirabilis var. maraisii (V.Poelln.) Bayer (2012): Haworthia maraisii V.Poelln. in Feddes Repert. 38:194 (1935): H. magnifica var. maraisii (V.Poelln.) Bayer (1977). Type (icono.): Swellendam, Marais in Swellendam 6410 (B).
= H. schuldtiana V.Poelln. (1937). H. schuldtiana var. robertsonensis V.Poelln. (1940). H. schuldtiana var. minor Triebn. et V.Poelln. (1940). H. schuldtiana var. subtuberculata V.Poelln. (1940). H. whitesloaneana V.Poelln. (1937): H. schuldtiana var. whitesloaneana (V.Poelln.) V.Poelln. (1940). H. schuldtiana var. sublaevis V.Poelln. (1940). H. schuldtiana var. simplicior V.Poelln. (1940). H. schuldtiana var. unilineata V.Poelln. (1940). H. sublimpidula V.Poelln. (1936). H. triebneriana var. diversicolor Triebn. et V.Poelln. (1939). H. angustifolia var. subfalcata V.Poelln. (1951), nom. inval. *H. calliantha
H. mirabilis var. meiringii (Bayer) Bayer (2012): H. maraisii var. meiringii Bayer in Haw. Hand.:134 (1976): H. magnifica var. meiringii (Bayer) Bayer (1977). Type: E of Bonnievale, Bayer in KG 224/70 (NBG). *H. meiringii
H. mirabilis var. mundula (Smith) Bayer (2012): H. mundula Smith in S. Afr. J. Bot. 12: 8 (1946): H. mirabilis subsp. mundula (Smith) Bayer (1976). Type: on Elim road, Smith 5479 (NBG).
H. mirabilis var. notabilis (V.Poelln.) Bayer (2012): H. notabilis V.Poelln. in Repert. Spec. Nov. Regni. Veg. 44: 134 (1938): H. maraisii. var. notabilis (V.Poelln.) Bayer (1976): H. magnifica var. notabilis Bayer (1977). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] (B)..
= H. intermedia V.Poelln. in Kakteenk. 9: 133 (1937).: H. maculata var. ntermedia (V.Poelln.) Bayer (1999). H. schuldtiana var. erecta Triebn. & V.Poelln. (1940). H. nitidula var. opaca V.Poelln., nom. nud. (1948).
H. mirabilis var. paradoxa (V.Poelln.) Bayer in Haw. Revis.: 112 (1999): H. paradoxa V.Poelln. (1933): H. maraisii var. paradoxa (V.Poelln.) Bayer (1976): H. magnifica var. paradoxa (V.Poelln.) Bayer (1977). Neotype, designated by Breuer & Metzing (1997): Riversdale, Ferguson s.n. (BOL).
= H. jakubii Breuer in Alsterworthia Int. Sp. Is. 7: 10 (2004). *H. bobii
H. mirabilis var. scabra (Bayer) Bayer (2012): H. heidelbergensis var. scabra Bayer in Haw. Revis.: 82 (1999): H. scabrida Breuer (2010). Type: Drew, Leeurivier, Bayer 1700 (NBG).
H. mirabilis var. splendens (Hammer & Venter) Bayer (2012): H. magnifica var. splendens Hammer and Venter in Cact. Succ. J. (US) 70: 180 (1998): H. splendens (Hammer & Venter) M.Hayashi (2000). Type: W Albertinia, Venter (NBG).
H. mirabilis var. sublineata (V.Poelln.) Bayer in Haw. Revis.: 113 (1999): H. triebneriana var. sublineata V.Poelln. (1938): H. sublineata (V.Poelln.) Breuer (2010). Neotype, designated by Bayer (1999): S Bredasdorp, Smith 3966 (NBG).
H. mirabilis var. toonensis (Bayer) Bayer (2012): H. heidelbergensis var. toonensis Bayer in Haw. Revis.: 83 (1999): H. toonensis (Bayer) Breuer (2010). Type: Heidelberg, Matjestoon, Smith 6797 (NBG).
H. mirabilis var. triebneriana (V.Poelln.) Bayer in Haw. Revis.: 113 (1999): H. triebneriana V.Poelln. (1936). Lectotype, designated by Bayer (1999): [unpublished illustration] (B).
= H. willowmorensis V.Poelln. (1937). H. triebneriana var. depauperata V.Poelln. (1938): H. depauperata (V.Poelln.) Breuer (2010). H. triebneriana var. multituberculata V.Poelln. (1938). H. triebneriana var. rubrodentata Triebn. et V.Poelln. (1939). H. triebneriana var. napierensis Triebn. et V.Poelln. (1939). H. triebneriana var. turgida Triebn. (1939). H. triebneriana var. subtuberculata V.Poelln. (1939). H. triebneriana var. pulchra V.Poelln. (1940). H. rossouwii V.Poelln. (1938). H. nitidula V.Poelln. (1939). H. triebneriana var. diversicolor Triebner & V.Poelln. in Feddes Repert. 47: 9 (1939).: H. diversicolor (Triebner & V.Poelln.) M.Hayashi (2010).
H. monticola Fourcade in Trans. Roy. Soc. S. Africa 21:78 (1937). Type: George and Uniondale districts, Fourcade 2498 (K).
= H. divergens Bayer (1976).
H. monticola var. monticola
= H. bronkhorstii M.Hayahi in Haworthiad 15: 16 (2001). *H. baviens *H. glabella
H. monticola var. asema Bayer in Haw. Revis.: 117 (1999): H. asema (Bayer) M.Hayashi (2000). Type: Calitzdorp, Besemkop, Venter 12 (NBG).
H. mucronata Haw. in Suppl. Pl. Succ.: 50 (1819). Lectotype, designated by Bayer (1999): [unpublished illustration] (K).
= H unicolor V.Poelln. (1937): H. unicolor var. unicolor (V.Poelln.) Bayer (1982). H. mclarenii V.Poelln. (1939). H. tradouwensis Breuer in Avonia 21: 57 (2003). *H. armata *H. confluens *H. montagua
H. mucronata var. mucronata
H. mucronata var. habdomadis (V.Poelln.) Bayer in Haw. Revis.: 120 (1999): H. habdomadis V.Poelln. (1938): H. inconfluens var. habdomadis (V.Poelln.) Bayer (1976). Neotype, designated by Breuer & Metzing (1997): Seweweekspoort, Barker & Lewis s.n NBG2764/32 (BOL).
H. mucronata var. inconfluens (V.Poelln.) Bayer in Haw. Revis.: 121 (1999): H. altilinea var. limpida f. inconfluens V.Poelln. (1938): H. mucronata var. limpida f. inconfluens (V.Poelln.) V.Poelln. (1940): H. inconfluens (V.Poelln) Bayer (1976): H. habdomadis var. inconfluens (V.Poelln) Bayer (1977). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] Triebner 1031 (B).
= H. bijliana var. joubertii V.Poelln. (1936). H. setata var. bijliana sv. joubertii (V.Poelln.) V.Poelln. (1938): H. setata var. joubertii (V.Poelln.) Jacobsen (1960). H. integra var. standeri Esterhuizen in Haworthiad 14: 21 (2000): H. standeri (Esterhuizen) M.Hayashi (2010). H. crystallina M.Hayashi in Haworthia Study 15: 116 (2006).*H. allomadis *H. calitzensis *H. rooibergensis *H. horrida *H. kotei
H. mucronata var. rycroftiana Bayer in Haw. Hand.: 54 (1976). Gouritz River between Vanwyksdorp and Herbertsdale, Bayer 1701 (NBG).
= H. integra V.Poelln. in Feddes Repert. 33: 239 (1933).
H. mucronata var. morrisiae (V.Poelln.) V.Poelln. in Feddes Repert. 49: 29 (1940): H. altilinea var. morrisiae V.Poelln. (1938): H. inconfluens var. morrisiae (V.Poelln.) Bayer (1976): H. habdomadis var. morrisiae (V.Poelln.) Bayer (1977). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] (B).
= H. sakai M.Hayashi in Haworthia Study 3: 13 (2000).
H. mutica Haw. in Revis.:55 (1821). Lectotype, designated by Bayer (1978): [image] (K; later published in Excelsa 8: 50 (1978). Epitype, designated by Breuer & Metzing (1997): NE Soetrivier Bridge, Bayer KG623/69 (NBG).
= H. otzenii Smith (1945). H. groenewaldii Breuer in Alsterworthia Int. 11: 15 (2011).
H. nortieri Smith in Jl. S. Afr. Bot. 12: 13 (1946). Type: Vanrhynsdorp, Smith 1676a (NBG).
= H. nortieri var. montana Smith (1950). H. nortieri var. giftbergensis Smith (1950): H. giftbergensis (Smith) Breuer (2010). H. agnis Battista in Alsterworthia Int. 2: 9 (2002). H. montana M.Hayashi in Haworthia Study 14: 14 (2005), nom. inval.
H. nortieri var. nortieri
H. nortieri var. albispina (M.Hayashi) Bayer (2012): H. albinspina M.Hayashi in Haworthia Study 8: 1 (2002). Type: E Laingsburg, Hayashi 02-48 (TUAT).
H. nortieri var. devriesii (Breuer) Bayer (2012): H. devriesii Breuer in Avonia 21: 47 (2003). Type: Prince Albert, Breuer 6930 (TUAT).
H. nortierii var. globosiflora (Smith) Bayer (1976): H. globosiflora Smith (1950). Type: Doornbosch, N Doorn River Bridge, Smith 7198 (NBG).
H. nortierii var. pehlemanniae (Scott) Bayer in Haw. Revis.: 129 (1999): H. pehlemanniae Scott (1982). Type: W Laingsburg, Scott 7450 (PRE).
H. outeniquensis Bayer in Haw. Revis.: 130 (1999). Type: Moerasriver, Venter 94/61 (NBG). *H. heroldia
H. parksiana V.Poelln. in Cactus J. 5: 34 (1936). Lectotype, designated by Breuer & Metzing (1997): [image, later published in Desert Pl. Life 10: 48 (1938)] (B).
H. pubescens Bayer in J. S. Afr. Bot. 38: 129 (1973). Type: Worcester, Sandberg Hills, Bayer 163 (NBG).
H. pulchella Bayer in J. S. Afr. Bot. 39: 232 (1973). Type: Touws River, Avondrust, Bayer 163 (NBG).
H. pulchella var. pulchella
H. pulchella var. globifera Bayer in Haw. Revis. :136 (1999): H. globifera (Bayer) M.Hayashi (2000). Type: SE Anysberg, Bruyns 7338 (NBG).
H. pygmaea V.Poelln. in Repert. Spec. Nov. Regni Veg. 27: 132 (1930). Neotype, designated by Breuer & Metzing (1997): hills E Great Brak, Fourcade 4759 (BOL).
= *H. asperata
H. pygmaea var. pygmaea
H. pygmaea var. acuminata (Bayer) Bayer (2012): H. retusa f. acuminata Bayer in Haw. Handb.: 53 (1976): H. retusa var. acuminata (Bayer) Bayer (1982): H. magnifica var. acuminata (Bayer) Bayer (1999). Type: N of Gouritzmond, Bayer in KG 311/7 (NBG). *H. acuminata.
H. pygmaea var. argenteo-maculosa (Smith) Bayer in Haw. Revis.: 138 (1999): H. dekenahii var. argenteo-maculosa Smith (1945): H. retusa f. argenteo-maculosa (Smith) Bayer (1976). Type: between Gouritz Bridge and Mossel Bay, Emett s.n. NBG68037 (NBG).
= H. silviae M.Hayashi in Haworthia Study 3: 13 (2000).
H. pygmaea var. dekenahii (Smith) Bayer (2012): H. dekenahii Smith in Jl. S. Afr. Bot. 10: 140 (1944): H. retusa var. dekenahii (Smith) Bayer (1982): H. magnifica var. dekenahii (Smith) Bayer (1999). Type: Farm Draaihoek, J. Dekenah 86 sub Smith 5489 (NBG).
H. pygmaea var. fusca (Breuer) Bayer (2012): H. fusca Breuer in Alsterworthia Int. Special Issue 7: 9 (2004). Type: W Albertinia, Breuer 8971 (GRA).
H. pygmaea var. esterhuizenii (M.Hayashi) Bayer (2013). H. esterhuizenii M. Hayashi in Haworthia Study 7: 14 (2002). Type: Albertinia, Hayashi 96-6 (TUAT) H. pygmaea var. vincentii (Breuer) Bayer in Nomenclator (2012): H. vincentii Breuer in Alsterworthia Int. Special Issue 7: 11 (2004). H. vincentii Breuer in Alsterworthia Int. Special Issue 7: 11 (2004). Type: NE Albertinia, De Vries 071 (GRA).
H. reticulata (Haw.) Haw. in Syn. Pl. Succ.: 94 (1812): Aloe reticulata Haw. (1804). Neotype, designated by Bayer (1972): Illustration in Curtis’ Bot.Mag.: t. 1314 (1811). Epitype, designated by Breuert & Metzing (1997): SW Worcester, Ribokkkop, Bayer 160 (NBG).
= Aloe pumilio Jacq. (1804). H. reticulata var. acuminata (1938). H. hurlingii var. ambigua Triebn. & V.Poelln. (1938). H. guttata Uitew. (1947). H. intermedia V.Poelln. (1937), syn. nov: H. magnifica var. intermedia (V.Poelln.) Bayer (1999).
H. reticulata var. reticulata
H. reticulata var. attenuata Bayer in Haw. Revis.: 140 (1999). Type: S Bonnievale, Smith 3979 (NBG). *H. oxygona?
H. reticulata var. hurlingii (V.Poelln.) Bayer in New. Haw. Hand.: 52 (1982): H. hurlingii V.Poelln. (1936). Lectoptype, designated by Breuer & Metzing (1997): [image, later published in Desert Pl. Life 10: 125 (1938)] (B).
H. reticulata var. subregularis (Baker) Bayer in Haw. Revis.: 141 (1999): H. subregularis Baker (1870). Lectotype, designated by Bayer (1999): Illustration in Saund. Ref. Bot.: t. 232 (1870).
= H. haageana V.Poelln. (1930). H. haageana var. subreticulata V.Poelln. (1937).
H. retusa (L.) Duval in Pl. Succ. Hort. Alenc.: 7 (1809): Aloe retusa L. (1753). Lectotype, designated by Scott (1985): Illustration in Commelin, Horti Med. Amstelod. 2: t. 6 (1701). Epitype, designated by Breuer & Metzing (1997): Blikbonnie, E Riversdale, Dekenah s.n. NBG144772 (NBG).
= H. foucheii V.Poelln. (1940). H. retusa var. multilineata Smith (1946): H. multilineata (Smith) Scott (1985). H. retusa var. solitaria Smith (1946): H. solitaria (Smith) Scott (1973). H. retusa var. densiflora Smith (1946). H. geraldii Scott (1965). *H. subretusa
H. retusa var. retusa
H. retusa var. longibracteata (Smith) Bayer (2012): H. longibracteata Smith in Jl. S. Afr. Bot. 11: 75 (1945): H. turgida var. longibracteata (Smith) Bayer (1999). Type: near Stilbaai, Dekenah 18 sub Smith 5378 (NBG).
H. retusa var. nigra (Bayer) Bayer (2012): H. mutica var. nigra Bayer in Haw. Revis.: 126 (1999). Type: Kransriviermond, Smith 5753 (NBG). *H. quimutica *H. chromutica
H. retusa var. suberecta (V.Poelln.) Bayer(2012)H. turgida var. suberecta V.Poelln. in Repert. Spec. Nov. Regni Veg. 44: 134 (1938): H. suberecta (V.Poelln.) Breuer (2010). Neotype, designated by Bayer (1999): Brandwacht, Bayer s.n. KG631/69 (NBG).
= H. turgida var. subtuberculata V.Poelln. (1938). H. turgida var. pallidifolia Smith (1946): H. pallidifolia (Smith) Breuer (2010). *H. rodinii
H. retusa var. turgida (Haw) Bayer (2012)H. turgida Haw. in Suppl. Pl. Succ.: 52 (1819). Neotype, designated by Breuer & Metzing (1997): Swellendam, Breede River Bridge, Bayer 2420 (NBG).
= H. laetivirens Haw. (1819). H. caespitosa V.Poelln. (1936). H. caespitosa f. subplana V.Poell. (1938). H. caespitosa f. subproliferans V.Poelln. *H. pseuda *H. reflexa
H. rossouwii V.Poelln. in Kakteenk. 7: 75 (1938). Lectotype, designated here: [unpublished image] (B).
= H. serrata Bayer in Jl. S. Afr. Bot. 39: 249 (1973). Type: Heidelberg, Oudekraalkop, Bayer 166 (NBG).
H. rossouwii var. rossouwii
H. rossouwii var. calcarea (Bayer) Bayer (2012)H. mirabilis var. calcarea Bayer in Haw. Revis.: 110 (1999): H. calcarea (Bayer) M.Hayashi (2000). Type: Bredasdorp, De Hoop, Burgers 1648 (NBG).
H. rossouwii var. minor (Bayer) Bayer (2012)H. heidelbergensis var. minor Bayer in Haw. Revis.: 82 (1999): H. rooivleiensis Breuer (2010). Type: Bredasdorp, Rooivlei, Bayer sub KG 36/70 (NBG).
H. rossouwii var. petrophila (Bayer) Bayer (2012): H. variegata var. petrophila Bayer in Haw. Revis.: 159 (1999): H. petrophila (Bayer) M.Hayashi (2000). Type: Renosterfontein, Burgers 2158 (NBG).
H. rossouwii var. elizeae (Breuer) Bayer in Haworthia Update 2.2: 153 (2006): H. elizeae Breuer (2003). Type: W Swellendam, Breuer 6936 (TUAT).
H. semiviva (V.Poelln.) Bayer in Haw. Handb.: 153 (1976): H. bolusii var. semiviva V.Poelln. (1938). Lectotype, desigtnated by Breuer & Metzing (1997): [image, later published in Succulenta (Netherlands) 22: 25 (1940)] (B). *H. sphaeroidea *H. victoria
H. springbokvlakensis Scott in Jl. S. Afr. Bot. 36: 287 (1970). Type: Springbokvlakte, Scott 245 (PRE).
H. transiens (V.Poelln) Bayer in Haworthiad 16: 66 (2002): H. cymbiformis var. transiens (V.Poelln.) Bayer (1976): H. planifolia var. transiens V.Poelln. (1938). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] (B).
= H. cymbiformis var. translucens Triebn. et V.Poelln. (1938). H. cymbiformis var. multifolia Triebn. (1938). H. cymbiformis var. brevifolia Triebn. et V.Poelln. (1938). *H. klipensis
H. truncata Schonland in Trans. Roy. Soc. S. Afr. 1: 391 (1910). Type: near Oudtshoorn, Britten (K).
= H. truncata f. tenuis V.Poelln. (1938): H. truncata var. tenuis (V.Poelln.) Bayer (1976). H. truncata f. crassa V.Poelln. (1938). H. truncata f. normalis V.Poelln. (1938). H. truncata var. minor Breuer in Avonia 21: 59 (2003).: H. papillaris Breuer (2010).
H. truncata var. truncata
H. truncata var. maughanii (V.Poelln.) Bayer in Haw. Revis.: 151 (1999). H. maughanii V.Poelln. (1932). Neotype, designated by Breuer & Metzing (1997): Calitzdorp, Malherbe s.n. NBG307/40 (NBG).
H. variegata Bolus in J. Bot. Soc. S. Afr.: 137 (1929). Type: Botterkloof, Mrs. E. Ferguson s.n. BOL18900 (BOL).
H. variegata var. variegata
H. variegata var. hemicrypta Bayer in Haw. Revis.: 158 (1999): H. hemicrypta (Bayer) M.Hayashi (2000). Type: NE lower slopes of Potberg, Burgers 2582 (NBG).
H. variegata var. modesta Bayer in Haw. Revis.: 159 (1999): H. modesta (Bayer) Hayashi (2000). Type: SW Kathoek, Bayer 2551 (NBG).
H. vlokii Bayer in Haw. Revis.: 160 (1999). Type: Swartberg Mts., Vlok sub Venter 91/2 (NBG).
H. wittebergensis Barker in Jl. S. Afr. Bot. 8: 245 (1942). Type : Witteberg, Pieterse sub NBG 68214 (NBG).
H. zantneriana V.Poelln. in Cactus J. 5: 35 (1936). Lectotype, designated by Breuer & Metzing (1997): [image, later published in Desert Pl. Life 9: 90 (1937)] (B).
H. zantneriana var. zantneriana
H. zantneriana var. minor Bayer in Haw. Revis.: 164 (1999): H. inspida Breuer (2010). Type: near Miller Station, Bayer 1702 (NBG).
II. Subgenus Hexangulares (Uitewaal) M.B.Bayer [as Uitewaal ex M.B.Bayer] in Haw. Handb.: 14 (1976). Type species: Haworthia coarctata Haw. [Lectotype, designated by Bayer (1976)]. ± 15 spp.
H. attenuata (Haw.) Haw. in Syn. Pl. Succ.: 92 (1812): Aloe attenuata Haw. (1804). Neotype, designated by Breuer & Metzing (1997): 20 km E Patensie, Sandland. Perry 660 (NBG).
= H. clariperla Haw. (1928): Aloe attenuata var. clariperla (Haw) Salm-Dyck (1834): H. attenuata var. clariperla (Haw.) Baker (1880): H. attenuata f. clariperla (Haw.) Bayer (1976). H. fasciata var. caespitosa Berger (1908). H. britteniana V.Poelln. (1937): H. attenuata var. britteniana (V.Poelln.) V.Poelln. (1937): H. attenuata f. britteniana [as britteniae] (V.Poelln.) Bayer (1976). H. attenuata var. odonoghueana et vars. linearis, uitewaaliana, deltoidea, minissima, inusitata Farden (1939).
H. attenuata var. attenuata
H. attenuata var. glabrata (Salm Dyck) Bayer (2012): Aloe glabrata Salm Dyck in Hort.Dyck.: 325 (1834): H. glabrata (Salm Dyck) Baker (1880). Neotype, designated by Smith & Greyling (1990): Illustration in Salm Dyck, Aloes Mesembr. 3: Aloe t. 7 [sect. 6: 13] (1840).
H. attenuata var. radula (Jacq.) Bayer in Haw. Revis.: 167 (1999): Aloe radula Jacq. (1804): H. radula (Jacq.) Haw. (1812). Type: Illustration in Jacq., Pl. Hort.Schoenbr. 4: t. 422 (1804). Epitype, designated by Breuer & Metzing (1997): 1.6 km from Hankey to Thornhill, Smith 3190 (NBG).
H. bruynsii Bayer in J. S. Afr. Bot. 47:789 (1981). Type: SE Steytlerville, Rossouw 456 (NBG).
H. coarctata Haw. in Philos.Mag. 64: 301 (1824). Neotype, designated by Breyer & Metzing (1997): 16 km from Grahamstown to Bathurst, Smith 7092 (NBG).
= H. chalwinii Marl.et Berg. (1906). H. reinwardtii var. conspicua V.Poelln. (1937). H. fallax V.Poelln. (1932): H. reinwardtii var. fallax V.Poelln. (1937). H. reinwardtii var. pseudocoarctata V.Poelln. (1940): H. coarctata var. haworthii f. pseudocoarctata (V.Poelln.) Resende (1943). H. coarctata var. haworthii Resende (1943). H. coarctata var. kraussii Resende (1943). H. reinwardtii var. committeesensis Smith (1943). H. reinwardtii var. huntsdriftensis Smith (1944). H. fulva Smith (1943). H. musculina Smith (1948). H. greenii var. silvicola Smith (1943).
H. coarctata var. coarctata
H. coarctata var. coarctata f. coarctata
H. coarctata var. coarctata f. greenii (Baker) Bayer in Haw. Revis.: 172 (1999): H. greenii Baker (1880): H. greenii subsp. coarctata var. greenii (Baker) Bayer (1973). Type: Cape, Cooper 1860 (K).
= H. peacockii Baker (1880). H. greenii f. bakeri Resende (1943). H. greenii f. minor Resende (1943).
H. coarctata var. adelaidensis (V.Poelln.) Bayer in Haw. Revis.: 172 (1999): H. reinwardtii var. adelaidensis V.Poelln. (1940): H. coarctata subsp. adelaidensis (V.Poelln.) Bayer (1973): H. adelaidensis (V.Poelln.) Breuer in Gen. Haw. 1: 7 (2010). Lectotype, designated by Breuer & Metzing 91997): [unpublished image] (B).
= H. reinwardtii var. riebeeckensis Smith (1944). H. reinwardtii var. bellula Smith (1945).
H. coarctata var. tenuis (Smith) Bayer in Haw. Revis.: 173 (1999): H. reinwardtii var. tenuis Smith (1948): H. coarctata ssp. coarctata var. tenuis (Smith) Bayer (1973): H. tenuis (Smith) Breuer (2010). Type: Cape, Alexandria Dist. Smith 3420 (NBG).
H. fasciata (Willd.) Haw. in Suppl. Pl. Succ.: 57 (1819): Apicra fasciata Willd. (1811): Aloe fasciata (Willd.) Salm Dyck (1937). Neotype, designated by Breuer & Metzing (1997): Hankey, Stayner s.n. NBG110360 (NBG).
= H. fasciata var. major Haw. (1819). Aloe fasciata var. major Salm Dyck (1837): H. fasciata var. major (Salm Dyck) V.Poelln. (1938). H. fasciata var. subconfluens V.Poelln. (1937): H. fasciata f. subconfluens (V.Poelln.) V.Poelln. (1938). H. fasciata f. ovatolanceolata V.Poelln. (1938). H. fasciata f. sparsa V.Poelln. (1938). H. fasciata f. variabilis V.Poelln. (1938). H. fasciata f. vanstaadenensis V.Poelln. (1938). H. browniana V.Poelln. (1937): H. fasciata f. browniana (V.Poelln.) Bayer (1976).
H. glauca Baker in J. Linn. Soc. Bot. 18:203 (1880). Type: Zuurberg Pass, Cooper (K).
= H. carrissoi Resende (1941).
H. glauca var. glauca
H. glauca var. herrei (V.Poelln.) Bayer in Haw. Hand.: 122 (1976): H. herrei V. Poelln. (1929). Neotype, designated by Breuer & Metzing (1997): Campherspoort, Barker 5069 (NBG).
= H. herrei var. depauperata V.Poelln. (1932). H. jacobseniana V.Poelln. (1937). H. eilyae V.Poelln. (1937). H. jonesiae V.Poelln. (1937). H. herrei var. poellnitzii Resende (1943). H. eilyae var. poellnitziana Resende (1943). H. eilyae var. zantneriana Resende (1943). H. armstrongii V.Poelln. (1937): H. glauca var. herrei f. armstrongii (V.Poelln.) Bayer (1976).
H. granulata Marloth in Trans. Ror. Soc. S. Afr. 2:39 (1910): H. venosa subsp. granulata (Marloth) Bayer (1976). Type: Verlatenkloof, Marloth 4217 (BOL).
= H. schoemanii M.Hayashi in Haworthia Study 9: 14 (2003).
H. koelmaniorum Oberm. & Hardy in Fl. Pl. Africa :f. 1502 (1967). Type: Groblersdal, Hardy & Mauve 2267 (PRE).
H. koelmaniorum var. koelmaniorum
H. koelmaniorum var. mcmurtryi (Scott) Bayer in Haw. Revis.: 181 (1999): Haworthia mcmurtryi Scott (1984). Type: Loskop, SW Dam, McMurtry 5247 (PRE).
H. limifolia Marloth in Trans. Roy. Soc. S. Africa 1: 409 (1908). Type: W Delagoa Bay, Marloth 4678 (PRE).
= H. limifolia var. diploidea Resende (1940). H. limifolia var. tetraploidea Resende (1940). H. limifolia f. marlothiana Resende (1941): H. limifolia var. marlothiana (Resende) Resende (1943). H. limifolia var. schuldtiana Resende (1940). H. limifolia var. stolonifera Resende (1940). H. limifolia var. stolonifera f. pimentelli Resende (1943). H. limifolia var. stolonifera f. major Resende (1943). H. limifolia var. keithii Smith (1950). *H. gideonii
H. limifolia var. limifolia
H. limifolia var. arcana G.F.Smith & N.R.Crouch in Bradleya 19: 119 (2001): H. arcana (G.F.Smith & N.R.Crouch) Breuer in Gen. Haw. 1: 7 (2010). Type: #
H. limifolia var. gigantea Bayer in Jl. S. Afr. Bot. 28: 215 (1962): H. gigantea (Bayer) M.Hayashi (2000). Type: Nongoma, Bayer 112 (PRE).
H. limifolia var. glaucophylla Bayer in Haworthia Update 2: 1 (2006): H. glaucophylla (Bayer) Breuer (2010). Type: Mpumalanga, Three Sisters, F. Venter 13700 (NBG).
H. limifolia var. ubomboensis (Verdoorn) Smith in Jl. S. Afr. Bot. 16: 3 (1950): H. ubomboensis Verdoorn (1941). Type: 16km S Stegi, Keith s.n. PRE26392 (PRE).
H. longiana V.Poelln. in Feddes Repert. 41: 203 (1937). Neotype, designated by Breuer & Metzing (1997): [unpublished image] (B).
= H. longiana var. albinota Smith (1948).
H. nigra (Haw.) Baker in J. Linn. Soc. Bot. 18: 203 (1880): Apicra nigra Haw. (1825). Neotype, designated by Breuer & Metzing (1997): Campherspoort, Barker 5099 (NBG).
= H. schmidtiana V.Poelln. (1929): H. nigra var. schmidtiana (V.Poelln.) Uitew. (1948). H. schmidtiana var. angustata V.Poelln. (1937): H. nigra var. angustata (V.Poelln.) Uitew. (1948). H. schmidtiana var. suberecta V.Poelln. (1937): H. nigra var. suberecta (V.Poelln.) Uitew. (1948). H. schmidtiana var. pusilla V.Poelln. (1938): H. nigra var. pusilla (V.Poelln.) Uitew. (1948). H. ryneveldii V.Poelln. (1939). *H. eonigra
H. nigra var. nigra
H. nigra var. diversifolia (V.Poelln.) Uitew. in Succulenta: 51 (1948): H. diversifolia V.Poelln. (1937): H. schmidtiana var. diversifolia (V.Poelln.) V.Poelln. (1938). Neotype, designated by Bayer (1999): Kruidfontein, Bruyns in KG435/75 (NBG).
= H. schmidtiana var. diversifolia f. nana V.Poelln. (1938): H. nigra var. diversifolia f. nana (V.Poelln.) Uitew. (1948).
H. nigra var. elongata (V.Poelln.) Uitew. in Succulenta: 51 (1948): H. schmidtiana var. elongata V.Poelln. (1938). Neotype, designated here: Slagtersnek, Van Jaarsveld & Marthinus 7913 (NBG).
H. pungens Bayer in Haw. Revis.:188 (1999). Type: Braamriver. Bruyns 7090 (NBG).
H. reinwardtii (Salm Dyck) Haw. in Saxifrag. Enum. 2: 53 (1821): Aloe reinwardtii Salm Dyck (1821). Neotype, designated by Scott (1981): Illustration in Salm Dyck, Aloes Mesembr. 1: Aloe t. 12 [sect. 6: 16] (1836). Epitype, designated by Breuer & Metzing (1997): hill above Ncera River Bridge, Smith 3563 (NBG).
= H. reinwardtii var. major Baker (1880). H. reinwardtii var. pulchra V.Poelln. (1937). H. reinwardtii var. archibaldiae V.Poelln. (1937). H. reinwardtii var. peddiensis Smith (1943). H. reinwardtii var. valida Smith (1943). H. reinwardtii var. grandicula Smith (1944). H. reinwardtii var. haworthii Resende (1943). H. reinwardtii var. triebneri Resende (1943).
H. reinwardtii var. reinwardtii
H. reinwardtii f. reinwardtii
H. reinwardtii f. chalumnensis (Smith) Bayer (1976): H. reinwardtii var. chalumnensis Smith (1943). Type: Chalumna, Smith 513 (NBG).
H. reinwardtii f. kaffirdriftensis (Smith) Bayer (1976): H. reinwardtii var. kaffirdriftensis Smith (1943). Type: Kaffirdrift, Smith 3364 (NBG).
H. reinwardtii f. olivacea (Smith) Bayer (1976): H. reinwardtii var. olivacea Smith (1944): H. olivacea (Smith) Breuer (2010). Type: Kaffirdrift, Smith 5260 (NBG).
H. reinwardtii f. zebrina (Smith) Bayer (1976): H. reinwardtii var. zebrina Smith (1944). Type: Kaffirdrift, Smith 5258 (NBG).
H. reinwardtii var. brevicula Smith in Jl. S. Afr. Bot. 10: 11 (1944): H. brevicula (Smith) Breuer in Gen. Haw. 1: 7 (2010). Type: Frazers Camp, Smith 3138 (NBG).
= H. reinwardtii var. diminuta Smith (1948).
H. scabra Haw. in Suppl. Pl. Succ.: 58 (1819). Lectotype, designated by Scott (1980): [illustration, later published in Cact. Succ. J. (Los Angeles) 52: 274 (1980)] (K).
= H. tuberculata V.Poelln. (1931). H. tuberculata var. acuminata V.Poelln. (1938). H. tuberculata var. sublaevis V.Poelln. (1938). H. tuberculata var. subexpansa V.Poelln. (1938). H. tuberculata var. angustata V.Poelln. (1940). H. scabra var. johanii M.Hayashi in Haworthiad 15: 16 (2001).: H. johanii (M.Hayashi) Breuer (2010).
H. scabra var. scabra
H. scabra var. lateganiae (V.Poelln.) Bayer in Haw. Revis.: 195 (1999): H starkiana var. lateganiae (V.Poelln.) Bayer (1976): H. lateganiae V.Poelln. (1937). Lectotype, designated by Breuer & Metzing (1997): [image, later published in Desert Pl. Life 9: 103 (1937): (B).
H. scabra var. morrisiae (V.Poelln.) Bayer in Haw. Hand.: 137 (1976): H. morrisiae V.Poelln. (1937). Lectotype, designated by Breuer & Metzing 91997): [image, later published in Kakteenk. & Kakteenfr. 1937: 132 (1937)] (B). *H. plettens
H. scabra var. starkiana (V.Poelln.) Bayer in Haw. Revis.: 197 (1999): H. starkiana V.Poelln. (1933). Lectotype, designated by Breuer & Metzing (1997): [unpublished image] (B).
= H. smitii V.Poelln. (1938).
H. sordida Haw. in Revis.: 51 (1821): Aloe sordida Schult. & Schult.f. (1829). Neotype, designated by Scott (1985): Illustration in Salm Dyck, Aloes Mesembr. 7: Aloe t. 1 [sect 7: 2] (1863).
= H. sordida var. agavoides (Zant. & V.Poelln.) Smith (1950): H. agavoides Zant. & V.Poelln. (1938).
H. sordida var. sordida
H. sordida var. lavranii Scott in Cact. Succ. J. (US) 53: 70 (1981): H. lavranii (Scott) Breuer (2010). Type: Perdehoek, Little Karoo, Hechter s.n. PRE61124 (PRE).
H. tessellata Haw. in Phil. Mag. 44: 300 (1824): . Aloe tessellata (Haw.) Schult. & Schult.f. (1829): H. venosa subsp. tessellata (Haw.) Bayer (1976). Lectotype, designated by Scott (1978): [illustration, later published in Cact. Succ. J. (Los Angeles) 50: 75 (1978) (K).
= H. parva Haw. (1824): Aloe parva (Haw.) Schult. & Schult.f. (1829): H. tessellata var. parva (Haw.) Baker (1880). H. tessellata var. inflexa Baker (1880). H. engleri Dint. (1914): H. tessellata var. engleri (Dint.) V.Poelln. (1938). H. pseudotessellata V.Poelln. (1929). H. tessellata var. tuberculata V.Poelln. (1936). H. minutissima V.Poelln. (1939): H. tessellata var. minutissima (V.Poelln.) Viveiros (194?). H. tessellata var. elongata Van Woerden (1940). H. tessellata var. simplex Resende & V.Poelln. (1942). H. tessellata var. stepheneana Resende & V.Poelln. (1942). H. tessellata var. luisierii Resende & V.Poelln. (1942). H. tessellata var. palhinhiae Resende & V.Poelln. (1942). H. tessellata var. velutina Resende & V.Poelln. (1942). H. tessellata var. coriacea Resende & V.Poelln. (1942): H. coriacea (Resende & V.Poelln) Breuer (2010). H. tessellata var. coriacea f. longior Resende & V.Poelln. (1942). H. tessellata var. coriacea f. brevior Resende & V.Poelln. (1942). H. tessellata var. obesa Resende & V.Poelln. (1942). H. venosa ssp. recurva sensu Bayer (1976). H. crousii [spahlm. crausii] M.Hayashi in Haworthiad 15: 16 (2001). *H. helensis *H. mediata
H. venosa (Lam.) Haw. in Saxifrag. Enum: 51 (1821): Aloe venosa Lam. (1873). Lectotype, designated by Scott (1978): Illustration in Commelin, Praeludia Bot.: t. 29 (1703). Epitype, desigtnated by Breuer & Metzing (1997): Swellendam, W of Breede River Bridge, Bayer 168 (NBG).
= Aloe tricolor Haw. (1804). = Aloe recurva Haw. (1804): H. recurva (Haw.) Haw. (1812). = H. distincta Brown (1876). = H. venosa var. oertendahlii Hjelmquist (1943). *H. irmiae
H. viscosa (L.) Haw. in Syn. Pl. Succ.: 90 (1812): Aloe viscosa L. (1753). Lectotype, designated by Scott (1981): Illustration in Commelin, Praeludia Bot.: t. 31 (1703). Epitype, designated by Breuer & Metzing (1997): Calitzdorp, Blackburn Valley, Barker 5073 (NBG).
= Aloe pseudotortuosa Salm Dyck (1817): H. pseudotortuosa (Salm Dyck) Haw. (1819): H. viscosa var. pseudotortuosa (Salm Dyck) Baker (1880). Aloe subtortuosa Salm Dyck (1836). Aloe tortuosa Haw. (1804): H. tortuosa (Haw.) Haw. (1812). H. concinna Haw. (1819): Aloe concinna (Haw.) Haw. (1829): H. viscosa var. concinna (Haw.) Baker (1880). Aloe tortuosa var. major Salm Dyck (1817). H. asperiuscula Haw. (1819): Aloe asperiuscula (Haw.) Salm Dyck (1836). H. cordifolia Haw. (1819): Aloe cordifolia (Haw.) Salm Dyck (1836). H. indurata Haw. (1821): Aloe viscosa var. indurata (Haw.) Salm Dyck (1836): H. viscosa var. indurata (Haw.) Baker (1880). H. viscosa var. major Haw. (1821). = H. viscosa var. minor Haw. (1821). H. viscosa var. parvifolia Haw. (1821). H. torquata Haw. (1827): Aloe torquata (Haw.) Salm Dyck (1836): H. viscosa var. torquata (Haw.) Baker (1880). H. viscosa var. subobtusa V.Poelln. (1938). H. viscosa var. caespitosa V.Poelln. (1938). H. beanii Smith (1945). H. beanii var. minor Smith (1945). H. viscosa var. cougaensis Smith (1945). H. viscosa var. viridissima Smith (1945). H. asperiuscula var. subintegra Smith (1945). H. asperiuscula var. patagiata Smith (1946). H. viscosa var. quaggaensis Smith (1948). H. viscosa var. variablis Breuer in Avonia 21: 61 (2003).: H. variabilis (Breuer) Breuer (2010).
H. woolleyi V.Poelln. in Repert. Spec. Nov. Regni Veg. 42: 269 (1937): H. venosa subsp. woolleyi (V.Poelln.) Bayer (1999). Lectotype, designated by Breuer & Metzing (1997): [image, later published in Cact. J. (Croydon) 7: 3 (1938) (B).
III. Subgenus Robustipedunculatae (Uitewaal) M.B.Bayer [as Robustipedunculares Uitewaal ex M.B.Bayer] in Haw. Handb.: 14 (1976). Type species: Haworthia margaritifera (L.) Haw. [lecto., designated by Bayer in Haw. Handb.: 14 (1976)]. 4 spp.
H. kingiana V.Poelln. in Cact. J. 5:31 (1936): H. subfasciata var. kingiana V.Poelln. (1938). Neotype, designated by Breuer & Metzing (1997): Great Brak, Dekenah 201 (NBG).
= H. zenigata M.Hayashi in Haworthiad 15: 20 (2001).
H. marginata (Lam.) Stearn in Cactus J. 12: 34 (1938): Aloe marginata Lam. (1783). Lectotype, designated by Scott (1985): Illustration in Commelin, Praeludia Bot.: t. 30 (1703).
= Aloe albicans Haw. (1804). H. albicans (Haw.) Haw. (1812). H. laevis Haw. (1821): H. marginata var. laevis (Haw.) Jacobson (1960). H. virescens Haw. (1821): H. albicans var. virescens (Haw.) Baker (1896): H. marginata var. virescens (Haw.) Uitew. (1939). H. ramifera Haw. (1821): H. marginata var. ramifera (Haw.) Jacobson (1960).
H. minima (Aiton) Haw. in Syn. Pl. Succ.: 92 (1812): A. margaritifera var. minima Ait. (1789). Lectotype, designated by Scott (1985): Illustration in Dillenius, Hort. Eltham: t. 16, f. 18 (1732).
= A. pumila var. margaritifera gamma L. (1753). Aloe margaritifera var minor Ait. (1789): Haworthia minor (Haw.) Duv. (1809). Apicra granata Willd. (1811): H. granata (Willd.) Haw. (1819): A. granata (Willd.) Schult. & Schult.f. (1829): H. margaritifera var. granata (Willd.) Baker (1880). A. margaritifera var. minor Ker‑G. (1811). A. erecta var. laetivirens Salm Dyck (1824). Aloe erecta Salm Dyck (1836). Aloe margaritifera var. major Ait. (1789): H. major (Ait.) Duv. (1809). A. margaritifera var media DC. (1799). H. brevis Haw. (1819): A. brevis (Haw.) Schult. & Schult.f. (1829). H. erecta Haw. (1819): A. erecta (Haw.) Schult. & Schult.f. (1829) [non A. erecta Salm Dyck (1836)]: H. margaritifera var. erecta (Haw.) Baker (1880). H. margaritifera var. corallina Baker (1880). H. mutabilis V.Poelln. (1938). H. opalina M.Hayashi in Haworthiad 15: 17 (2001). *H. flavens *H. obrata
H. minima var. minima
H. minima var. poellnitziana (Uitew.) Bayer in Haw. Revis.: 213 (1999): H. poellnitziana Uitew. (1939). Type: Drew, Meiring (AMD).
H. pumila (L.) Duval. (1809): Aloe pumila L. (1753): Aloe pumila var. margaritifera L. (1753): Aloe margaritifera (L) Burm.f (1768): H. margaritifera (L.) Haw. (1819). Lectotype, designated by Scott (1985): Illustration in Commelin, Horti Med. Amstelod.: t. 10 (1701).
= Aloe arachnoidea var. pumila Ait. (1789): A. pumila (Ait.) Haw. (1804), hom. illegit. non A. pumila L. (1753). Aloe margaritifera var. maxima Haw. (1804): H. maxima (Haw.) Duv. (1809): A. semimargaritifera var. maxima (Haw.) Salm Dyck (1817). H. margaritifera var. semimargaritifera (Salm Dyck) Baker (1880). A. papillosa Salm Dyck (1817): H. papillosa (Salm Dyck) Haw. (1819). *H. sparsa.
Hybrids
H. mortonii Breuer in Alsterworthia Int. 7: 22 (2007). *H. hammeri