44. Haworthia coarctata Haw., Phil.Mag. 44:301(1824). Baker, JLinn.Soc.Bot. 18:202(1880). Salm Dyck, Monogr. 6:f17(1936). V.Poelln., Feddes Rep.Spec.Nov. 43:108(1938). Bayer, Natn.Cact.Succ.J 28:80(1973): Bayer :107(1976). Bayer :64(1982). Scott :48(1985). Type: Not preserved. Neotype (designated here): icon (K). Epitype (ex B&M): Grahamstown to Bathurst, Smith 7092 (NBG). H. chalwinii Marl.et Berg., Notizbl.Bot.Gart.Mus.Berl. 4:247(1906). Type: Cape, Graaff Reinet, Marloth 4051 (K): H. reinwardtii var. conspicua V.Poelln., Feddes Repert.Spec.Nov. 41:210(1937). Type: Cape, Port Elizabeth to Alexandria road, Archibald 85 in Long 346. Not preserved: H. reinwardtii var. fallax idem :209(1937). H. fallax idem. 31:83(1932). Type: Cape, Grahamstown, STE6633. Not preserved: H. reinwardtii var. pseudocoarctata V.Poelln., Beitr.Sukk. 2:43(1940). H. coarctata var. haworthii fa pseudocoarctata (V.Poelln.) Res., Mems.Soc.Broteriana: Succ.Afr. 3:84(1943). H. coarctata var. haworthii Res. ibid.: H. coarctata var. kraussii ibid.: H. reinwardtii var. committeesensis Smith, JS.Afr.Bot 9:93(1943). Type: Cape, Albany Div. Smith 551 (NBG): H. reinwardtii var. huntsdriftensis ibid. 10:14(1944). Type: Cape, Albany Div., Smith 3849 (NBG): H. fulva idem. 9:101(1943). Type: Cape, Bathurst Dist., Smith 3380 (NBG): H. musculina ibid. 14:49(1948). Type: Cape, Bathurst Dist., Smith 5118 (NBG): H. greenii var. silvicola ibid. 9:103(1943). Type: Cape, Bathurst Dist., Smith 3378 (NBG).
coarctata: leaves pressed together.
Rosette to 120mm φ, caulescent, proliferating. Leaves many, to 70 X 20mm, ratio stem diameter to leaf width 1:1.7, erect spreading or incurved, scabrid, brownish-green, usually with rounded tubercles. Inflorescence simple or occasionally compound, to 300mm. Flowers tepals fused, tube straight, lower inner tepals revolute.
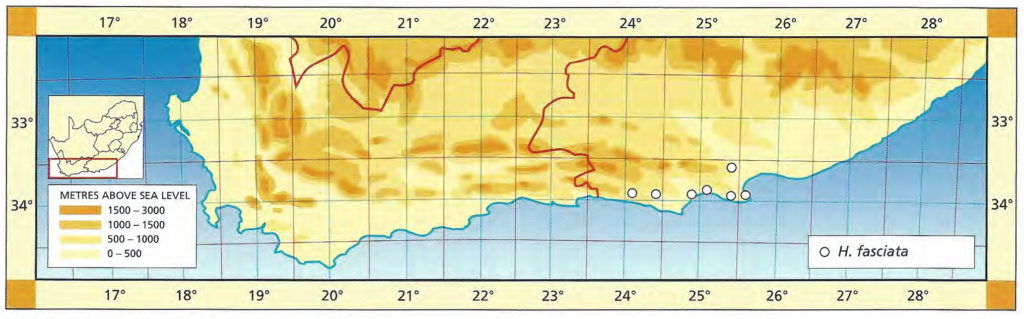
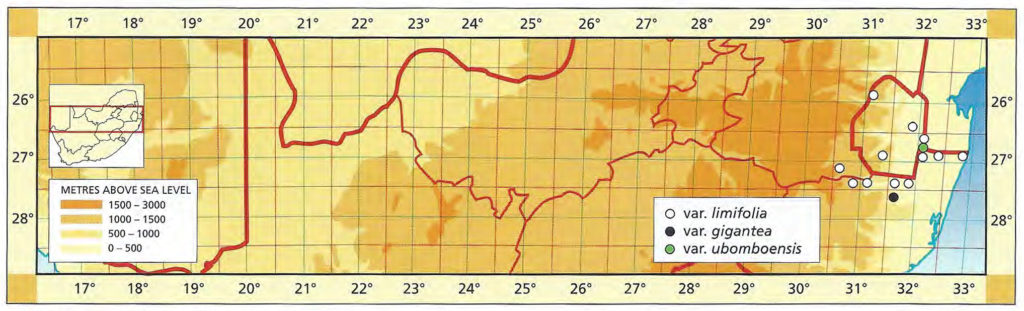
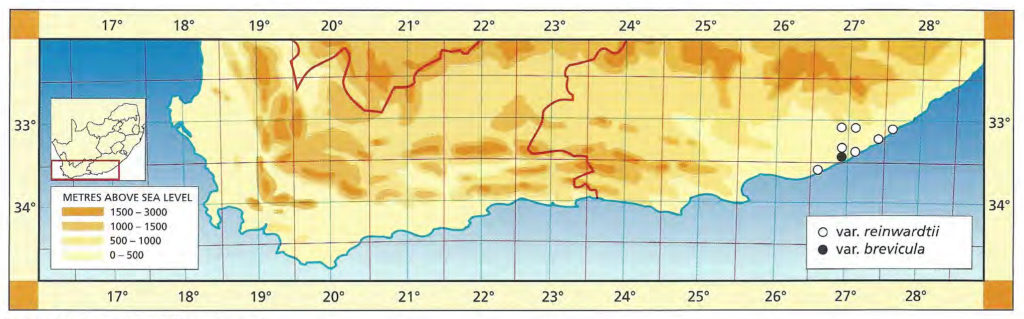
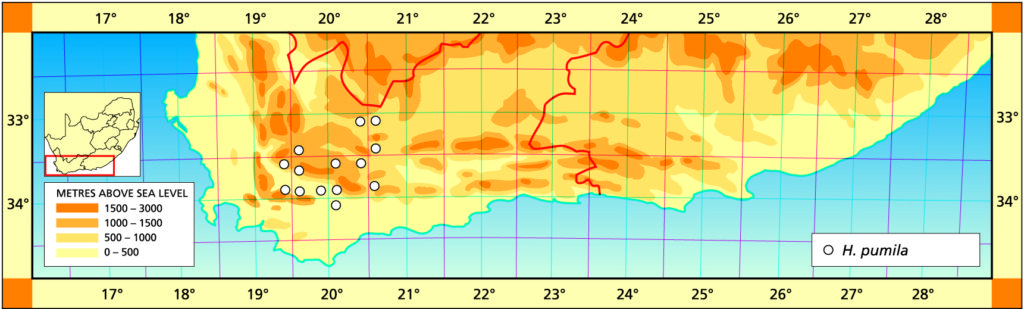
1982 – This is one of the stem‑forming species occurring west of the Fish River north of Grahamstown and extending to near Port Elizabeth. It has very often been confused with H. reinwardtii as the above synonymy suggests. However, there is a distinction based on both geographical distribution and morphological discontinuity. This is discussed in detail in Bayer (1973). Essentially there is a difference between the two species reflected in the number of leaves per unit length of stem. In H. coarctata the ratio of stem diameter to leaf width is 1:1.7 while in H. reinwardtii leaf width more nearly equals stem diameter and the ratio is 1:1.2. In H. coarctata the leaves are less densely arranged on the stem and also thicker. Another difference is that in H. coarctata the tubercles are smaller and more smoothly rounded, whereas in H. reinwardtii they are frequently large and tend to be flattened and whiter. H. coarctata has many striking forms and, possibly because of vegetative reproduction, populations tend to be very uniform. The subspecies coarctata contains the var. greenii, which is a glabrous variety occurring only at Howiesonspoort. The var. tenuis is a very slender variation with grossly elongated stems occurring near Alexandria in the south. There are countless other forms such as that which may have been described as H. chalwinii from along the Kowie River; the beautifully silvery tubercled forms from the Salem area and the very large hexaploids from the western and northern populations. The subsp. adelaidensis is a smaller element which occurs in the False Macchia around Grahamstown, perhaps the counterpart of H. reinwardtii var. brevicula towards the Fish River. The var. bellula must have been an aberrant clone because no such really small forms were found on a visit to the type locality. The western limit of H. coarctata is not known but it appears to be before the 26th line of longitude, east of Paterson, where the plants are still large and probably hexaploid. Odd and unconfirmed collections from between here and Uitenhage, notably at Addo, suggest that depauperate forms may be found in that area.
1999 – The changes here are the abandonment of the sub-species in favour of varietal rank. Possibly the correct solution is actually the inclusion of H. coarctata and H. reinwardtii in a single species. It is sometimes difficult to separate the two elements and there is an indication of their single identity in the Port Alfred to Alexandria vicinity. There may also be a similar problem in the area above and to the west of Hunt’s Drift. This is where the var. huntsdriftensis was collected and I was unable to find anything other than H. reinwardtii var. brevicula in that general area. While many species and varieties have been named within this group it is obvious from those and from new collections, that an endless array of different forms could be selected. This is in fact what G.G. Smith was in the process of doing as testified by the species fulva and musculina and greenii var. silvicola from the one farm, Hopewell. Smith also identified plants from this farm as H. chalwinii and the typical H. coarctata. A similar situation occurred with the Kaffirdrift populations of H. reinwardtii. The species citations show that Smith collected and numbered individual clones. During the years between collection and the making of the herbarium specimen, these plants were observed and described in minute detail. It is obvious that Smith was in the process of identifying new species and varieties from this array of material. H. chalwinii was described from a plant sent by Marloth from Graaff-Reinet. This is a form of H. coarctata which has a much narrower rosette with shorter stubby leaves. I have collected similar forms along the Kowie River, and Smith’s identification for Hopewell is not the only evidence suggesting occurrence in other populations. The most interesting new record is that by Mr Massyn in the Motherwell area of Port Elizabeth. This record is of a large form refuting the earlier suggestion of depauperate forms to the west. The distribution of the species is still complementary to that of H. fasciata, however, and fuller distribution records of both species are still necessary.
Dr D. Cutler (priv.comm.) said that he did not support he differences between H. coarctata and H. reinwardtii as set out by me in 1973. However, there is no evidence against my suggestion even though Col. Scott also disregards that arrangement altogether. If these two are correct, then the rational alternative is that only one species is involved. This is a solution which I would accept with the proviso that the geographical subdivision of that species is possible. The vars coarctata and adelaidensis are in two cases, viz. Hellspoort and Gowies Farm, reported from the same locality, although the latter identification is from herbarium specimens only. H. baccata is a name given by Smith to a plant ostensibly from southwest of Stutterheim (Smith 3572, wrongly given as 3782 by myself in 1982, and as 3527 by Scott 1985). The NBG herbarium specimen is annotated by McClaren as an apparent introduction to the area. It was never seen there by Smith and on visiting the given site, I could neither find it nor concede that the species could grow there. I noted in 1982 that a specimen noted by me as Smith 3782, was also in the Berlin Dahlem herbarium, which did not accord with the NBG specimen. Confusion is very easily sown.
a.var. coarctata
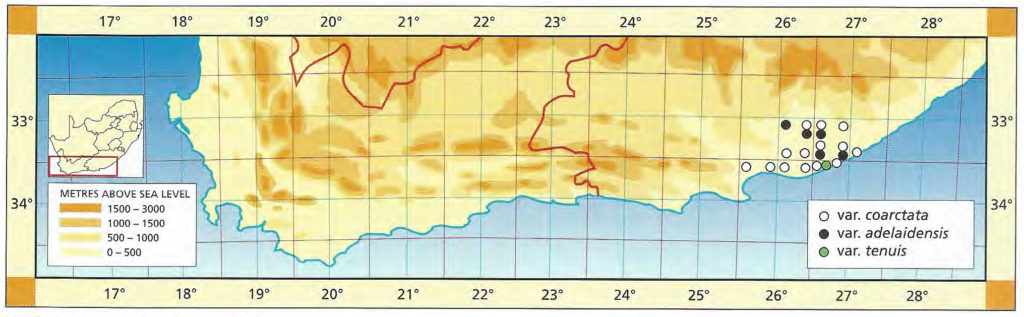
This is the main body of the species west of the Fish River. Three taxonomic variants are recognised as is evident below. Of these, the fa greenii is very localised. The two varieties have more substance, but the typical variety still encompasses a substantial range of variation. Again I see no reason to depart from the stable historical record of the species to find a neotype.
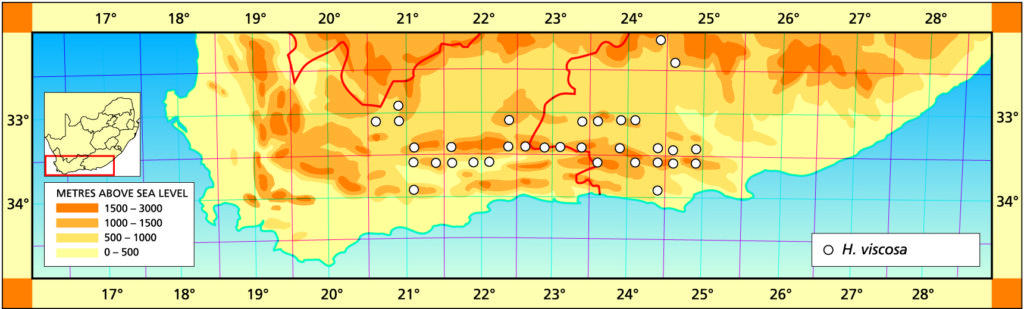
Distribution: 3325 (Port Elizabeth): Motherwell (-DA), Massyn (NBG); Addo (-DA), Fourcade 39 (NBG), Smith 2774 (NBG); Orlando (-DB), Smith 5348, Bayer 1371 (NBG). 3326 (Grahamstown): Hellspoort (-AB), Bayer 1352 (NBG); Piggot Bridge road (-AB), Britten in PRE 34837; Bushmans River Poort, Paynes farm (-AC), Archibald 5595 (PRE); Rockdale, Highlands (-AC), Dyer 2213 (BOL); Salem to Alexandria (-AD), Smith 3573, 3955, 4010 (NBG), Stayner (NBG); NE. Salem (-AD), Smith 3412, 3414, 3415 (NBG); Yellowwoods (-AD), Bayer 1372 (NBG); Howiesonspoort (-AD), Dyer 2507, 2508 (BOL), Reynolds in NBG987/34 (NBG); Rooidrift (-BA), Bayer 1367 (NBG); Fort Brown (-BA), Walton in NBG141/24 (BOL), Smith 5825 (NBG), Walton in NBG141/24; Gowie’s Farm (-BA), Smith 5625a (NBG); E. Plutosvale (-BA), Bayer 1368 (NBG); Fletcher’s Farm (-BA), Smith 5423, 5424, 5425 (NBG); E. Committees (-BB), Smith 7421 (NBG); W. Committees (-BB), Smith 551 (NBG, PRE); W. Breakfast Vlei (-BB), Smith 3959 (NBG); Brooklands (-BC), Bayer 1357 (NBG); Manley Flats (-BC), Fourcade 195, Bayer 1358 (NBG); Vaalvlei (-BC), Bayer 1360 (NBG); Hills SE. Grahamstown (-BC), Britten in PRE 34834; 11km Grahamstown to East London (-BC), Wells 2661 (PRE); SE. Grahamstown (-BC), Smith 7092, 7092a,b,c,d,f,h (NBG); W. Hunt’s Drift (-BD), Smith 3849 (NBG ,PRE), Smith 6818, 6818a,b (NBG); Bathurst (‑BD), Smith 7092 (NBG); Woodbury (CA), Bayer 1370 (NBG); Salem to Alexandria (-CB), Smith 3413, 3410, 3411 (NBG), Bayer 1363 (NBG); 24km W. Port Alfred (-DA), Smith 3378 (PRE); Kowie (-DA), Bayer 1359 (NBG); S. Vaalvlei (-DA), Bayer 1361 (NBG); Hopewell, 20km W. Port Alfred (-DA), Fourcade 119 (NBG), Smith 3380 (NBG, PRE), Smith 3378, 5114, 5115, 5116, 5117, 5118 5119 5120 (NBG), Bayer 1364, 1366; 24km WNW. Port Alfred (-DA), Smith 5115 (PRE); Near Ghio Bridge (-DA), Smith 7342 (NBG); Hopewell (-DA), Acocks 11054 (PRE); Glennismoyle (-DB), Smith 3416, 7491 (NBG) Fairfax (-DB), Bayer 1194 (NBG). 3327 (East London): E. Hunt’s Drift (-AC), Smith 5667 (NBG).
Inadequately located: Albany, Blackbeard in NBG 582/25; Brakfontein, Alexandria, Galpin (BOL); Alexandria, Archibald in NBG1322/37 (NBG); ex hort Marloth 4215 (PRE), Repton 61 (PRE), Whitehill (NBG).

Haworthia coarctata var. coarctata JDV90/46 Howiesonspoort. The density of leaf arrangement and tubercle pattern are a little different in each population. 
Haworthia coarctata var. coarctata JDV97/76 Belmont, east of Grahamstown. Often in huge mats with individual stems to 30cm.
b. fa greenii (Baker) Bayer comb.nov. H. greenii ssp. coarctata var. greenii (Baker) Bayer, Natn.Cact.Succ.J 28:80(1973). Bayer :120(1976). Bayer :65(1982). H. greenii Baker, JLinn.Soc. 18:202(1880). Scott :50(1985). Type: Cape, Cooper 1860 (K): H. peacockii Baker, JLinn.Soc. 18:202(1880). Type: ex hort. Kew. Not preserved: H. greenii fa bakeri Res., Mems.Soc.Broteriana: Succ.Afr. 3:87(1943). ex hort. Lisbon. Not preserved: H. greenii fa minor ibid. Type: ex hort. Lisbon. Not preserved.
greenii: for C.G. or G.H. Green.
This variant is only known from Howiesonspoort, and the normal tubercled variety is also present both to the east and west of the specific site of this glabrous variant. Col. Scott suggests a wider distribution but there is absolutely nothing in the herbarium record to support any notion that this is an independent entity. Even the criteria (no tubercles) is really inconsequential in a genus where many species may have tubercled or non-tubercled variants. The var. silvicola is obviously also an insignificant variant from the farm Hopewell where Smith collected many variants which he translated into species and varieties.
Distribution: 3326 (Grahamstown): Howiesonspoort (‑AD), Howiesonspoort, Dyer 2506, Reynolds in NBG 68038, Reynolds in NBG987/34, (BOL, NBG), Dyer 2507, 2508 (NBG), Smith 3178, 3524, 3525 5303 (NBG).
Inadequately located: ex hort. Whitehill, Smith 7448 (NBG).

Haworthia coarctata var. coarctata fa. greenii JDV90/46 Howiesonspoort. This form has no tubercles and its distribution is limited to a small area west of Grahamstown.
c. var. adelaidensis (V.Poelln.) Bayer comb.nov. H. coarctata ssp. adelaidensis (V.Poelln.) Bayer, Natn.Cact.Succ.J 28:86(1973). Bayer :94(1976). Bayer :65(1982). H. reinwardtii var. adelaidensis V.Poelln., Beitr.Sukk. 2:43(1940). Type: Cape, Adelaide, Armstrong. Not preserved. Neotype (B&M): icon (B): H. reinwardtii var. riebeeckensis Smith, JS.Afr.Bot. 10:16(1944). Type: Cape, Albany Div., Smith 5218 (NBG): H. reinwardtii var. bellula ibid. 11:70(1945). Scott, Nat.Cact.Succ.J 36:37(1981). Scott :47(1985). Type: Cape, Albany Div., Smith 3137 (NBG).
adelaidensis: from Adelaide, Cape.
As noted above there are two records which indicate that the typical variety, and this one, occur in close proximity. This may also be true for a site to the southeast of Grahamstown. The variant bellula is obviously a single clone which was collected my Miss Britten. A second clone was annotated ‘long-leaves’. Smith himself subsequently appears to have visited the site, or at least the near vicinity, twice. He does not record collecting a similar plant, nor do his specimens show this. Both myself and G. Marx have failed to find a similar clone at the given site. There is very little difference between this variety and var. tenuis except that the latter does develop much longer stems with age.
Distribution: 3326 (Grahamstown): 8km W. Riebeek East (-AA), Smith 7399 (NBG); Willowfountain (-AA), Bayer 1355 (NBG); Near Riebeek East (-AA), Smith 5218 (BOL, NBG, PRE); Brakkloof (-AB), Marx 43 (NBG); Hellspoort (-AB), Smith 355 (NBG, PRE), Bayer 1351 (NBG); Top Plutosvale (-BA), Smith 5426, 5427, 5428, 5429, 5430 (NBG); Glendew (-BA), Smith 5421 (NBG); Gowie’s Farm (-BA), Smith 5625 (NBG); Striata Hill (-BA), Smith 2431 (NBG); Bothas Hill (-BA), Barnes (BOL); Near top Queens Road (BA), Smith 2839 (NBG, PRE); Commonage (-BC), Smith 7448 (NBG); Queens Road (‑BC), Smith 2839 (NBG), Smith 7427 (NBG); NW. Grahamstown (-BC), Smith 3137 (BOL, NBG), Smith 3137a, 5626, 5626a (NBG), Bayer 1354 (NBG); Ainsley’s Farm, Trappes Valley (-BD), Smith 6782 (NBG).
Inadequately located: Albany, Luyt in NBG307/45 (NBG); Henderson 1450 (NBG), Rennie in NBG 1268/32.

Haworthia coarctata var. adelaidensis JDV94/53 northwest of Grahamstown. The small form named as bellula. 
Haworthia coarctata var. adelaidensis JDV97/65 northeast of Grahamstown. The plants do vary in size but stem-length is usually restricted.
d. var. tenuis (Smith) Bayer comb.nov. H. coarctata ssp. coarctata var. tenuis (Smith) Bayer, Natn.Cact.Succ.J 28:80(1973). Bayer :106(1976). Bayer :65(1982). H. reinwardtii var. tenuis Smith, JS.Afr.Bot. 14:51(1948). Type: Cape, Alexandria Dist. Smith 3420 (NBG).
tenuis: slender.
This variety is generally about half the diameter of the more typical variety and the leaves are more slender – which would separate it from the fa chalwinii. There do appear to be other populations of such slender leaved forms along the lower Bushman’s River, which should be distinguishable from H. reinwardtii by both tubercle character and leaf density on the stem.
Distribution: 3326 (Grahamstown): Harvestvale, Alexandria (‑DA), Smith 3417, 3418, 3419, 3420 (NBG), Holland 3817 (BOL).
Inadequately located: Bushmans River, Britten (NBG).

Haworthia coarctata var. tenuis. This relatively delicate variant is prone to die back at the leaf tips unless it is evenly watered or well shaded. 
Haworthia coarctata var. tenuis PVB Harvestvale. Mat forming with long slender stems which are very similar to var. adelaidensis, but grow very much longer.