M. B. Bayer (MSc), Kleinbegin Farm, Kuilsriver, South Africa
Mrs. Carly Cowell (MSc), Regional Ecologist, Cape Research Centre, Conservation Services, South African National Parks, Cape Town, South Africa.
Objective: The significance of the Bontebo National Park occurrences.
The occurrence of members of three aloid “genera” (the three sub-genera of the genus Haworthia could indeed be genera) and the absence of any other member of the Aloids (bar the ubiquitous Aloe ferox) must surely be indicative of the driving forces that determine the flora of the Bonte National Park. This also must surely help establish the significance of the park as a conservancy of considerable merit. The complex interaction of the species enhances even that. The purpose of this report is to examine more closely the variation and nature of a small segment of the Park flora, and demonstrate how much more can be done.
Note: This report has several constraints. Firstly is the situation in which there is no formal general definition and hence understanding of what a plant species is. Secondly there is the generally understood view that there is an evolutionary process at work by which organisms evolve from a common distant origin by genetic mutation and adaptation. Thirdly there are serious flaws in the classification of the Aloid genera. Several essays dealing with these issues by DNA sequencing are weak because they rest on those flaws and consequently do not address some serious questions of relationships that the results pose. Fourthly of course is the reality that the knowledge or intellectual capacity to overcome these deficiencies may be absent. Thus the report is written in the context of all the publications as the original genus revision (Haworthia Revisited, Bayer; Umdaus, 1999) and others available on the internet (HaworthiaUpdates.org).
Introducing the subgenera.
There are 6 species of Haworthia that occur in the Bontebok National Park representing each of the three subgenera. These subgenera are not real and have no closer relationship than that between any of the other genera in the Asphodelaceae however these are, or have been recognised.
Subgenus Hexangulares. There are 15 species in this subgenus. Two are in the northwest of South Africa and the others in the Eastern Cape or across the Karoo. In respect of other Aloid genera it seems to be close to Chortolirion. Only one species occurs in the Southern cape viz.H. venosa.
Subgenus Haworthia. Here there are 37 species by my account. Non-botanists raise this figure to as much as 600. By their methodology and ideology it is a great deal more. This subgenus is at odds with all the other Aloid genera and has an unusual internal diversity suggesting very recent speciation. Three species occur in the Park viz. H. retusa (turgida), H. mirabilis and H. floribunda.
Subgenus Robustipedunculares. There are 4 species all in the Southern cape with spill-over into the Little Karoo. One species, H. pumila also occurs in the southern Great Karoo. There is a very close affinity with the genus Astroloba. The four species have a very close affinity and while H. kingiana in the eastern Southern Cape appears to be discrete, the other three definitely are not. The Park occurrences are evidence of this and these seem to represent two species viz. H. marginata and H. minima. See more under the treatment of H. minima below.
Introducing the species.
Illustrations are of a general form for the species. Clonal variability is so high that use of the term “typical” can be misleading.
Haworthia venosa. (Fig. 1)
This species occurs primarily along the lower course of the Breede River with its upper river limit just beyond the northwestern corner of the Bontebok National Park. Its lower limit is just north of Malgas. Two populations are known to me off the river and a small way to the west. The species also occurs at Die Eiland in the Gouritz Valley. Its nearest affinities are with H. tessellata that is very widespread across the northern Karoo and Cape; and with H. granulata. The latter is certainly more closely allied to H. tessellata and the separation may just be on the basis of geographic separation rather than on the morphological characters that could be cited. This may be true for H. venosa as well but in my opinion this would conflict with the general evidence of species geography.

Haworthia retusa (turgida). (Fig. 2)
The name is written in this form because in the existing situation where species are confounded by definition, lesser categories are even more so. The bracketing thus suggests that this is a variant of H. retusa. This species is very widespread and abundant east of the Park with one vicariant population just north of Bredasdorp. The (turgida) variant is one I regard as a riverine cliff ecotype and occurs in the park only along the Breede River at the northwest limits of the Park. It is also downstream along the Breede River. H. mutica is a closely related species that in my opinion actually just extends the distribution of H. retusa (retusa) westwards. My opinion further is that an “evolutionary” process is not complete and that H. retusa and H. mirabilis may be the same “species” separated primarily on the basis of a different flowering season. H. mutica does occur just east of the Park (Rotterdam, Mullershof), and my observation is that H. floribunda is involved with H. retusa and H. mirabilis in this occurrence. It is in fact one of the objectives in this report; to ask if this might be true. Flowering time is spring.
This species and its variants appears in various of my publications and often linked to H. mutica and H. pygmaea.
This species is very common and widely distributed from Genadendal, Caledon and Napier all the way to east of Albertinia. It is generally summer flowering and extremely diverse. As its interaction with H. retusa in the west generates H. mutica, so I believe the same interaction produces H. pygmaea in the east. H. retusa is spring flowering but hybrids with H. mirabilis are present in the field and there are populations that suggest that these two species are involved. Flowering time is summer.
Extensive records are discussed and illustrated in various publications but primarily in the Update series, specifically Update Vol.3, part 1. The species is incredibly variable and the floral variations are discussed and illustrated in a flower report as Haworthia Update Vol.8 (Alsterworthia 2012).

Haworthia floribunda. (Fig. 4)
This species is also summer flowering and has a curious relationship with both H. retusa and with H. mirabilis also with evidence that such “introgression” is evidenced in populations. It is at its western limit at Swellendam and the variants in this area are extraordinary. Flowering time is summer.
Also incredibly variable with hybridization with both H. mirabilis and H. retusa and it is hypothesized that some Haworthia populations are derived from such introgression (see also Haworthia Updates Vol.5 ,part 1, Chapter 6).
This species occurs from Ashton eastward to east of Riversdale in a rather narrow eas-west band that widens near Bredasdorp. It interacts with both H. pumila and H.minima. The species in this subgenus flower essentially in mid-summer. The Park population together with that of the adjacent Rotterdam population flowers in spring. At Rotterdam there is hybridization with a version of H. minima.
This species distribution generally complements that of H. pumila and then follows, but more widely that of H. marginata. In the southwest it extends to near Elim and is common along the near-coastal strip as far east as Hartenbos. The inland distribution is not well known in the area west of the Breede River. It is probable that it was common in the area now extensively cultivated but it is not known to me inland in that area north of Tarentaal. West of Swellendam there are only two records known to me at Sanddrift, Drew and again furher west of Drew. In the one case there is hybridization with H. marginata. In the second case a short distance west of this is the variant ‘poellnitziana’ and I suspect that introgression with H. pumila may have occurred there The essential differences are that H. pumila has a colour range in the brown-green range, the flowers are generally olivaceous, and the tubercles are larger and rougher. The colour range for the smaller H. minima plants is in the blue-green range, the tubercles are smaller and smoother, and the flowers whiter and greener. H. pumila hybridizes naturally with Astroloba corrugata. Also at Sanddrift, H. pumila hybridizes with H. marginata . It is almost inconceivable that it has not hybridized with H. minima and it will be very difficult to superficially judge if plants were indeed hybrids or not without resorting to DNA sequencing (when that technology is advanced enough). H. minima hybridises with H. marginata at Heidelberg (S. Hooikraal) and in the past with H. marginata just northeast of Bredasdorp. Flowering time is summer.
Some variation is presented and discussed in Haworthia Updates Vol.5. part 1, Chapter 7.
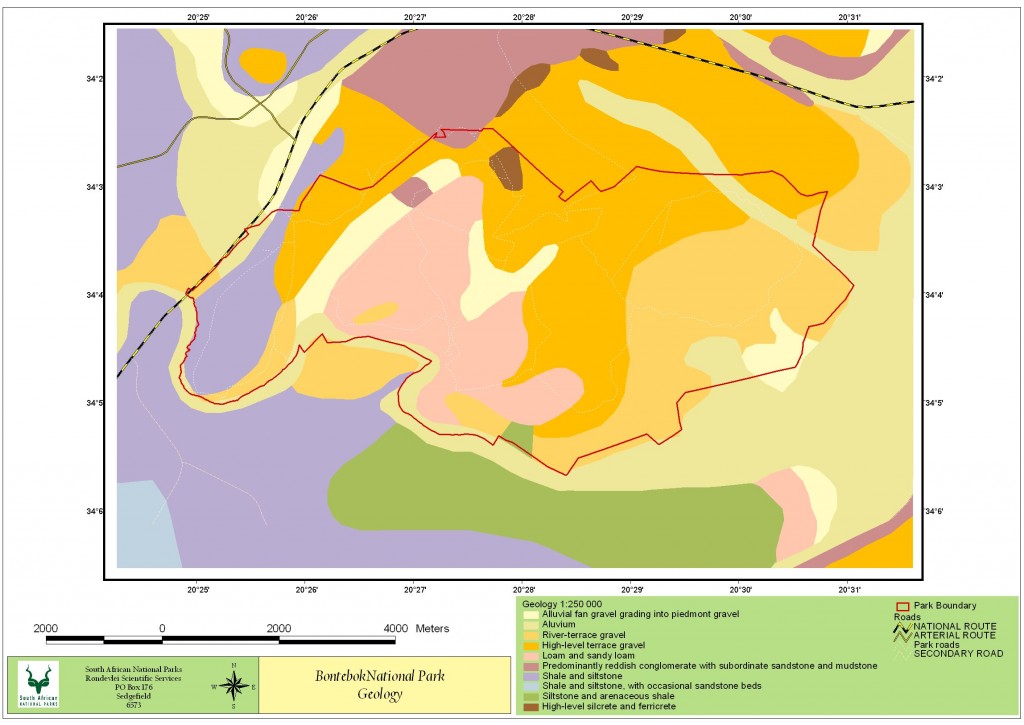
The geology of the Park
Bontebok National Park (BNP) is home to three main land types listed in the National Land Type Database, the first land type consists of conglomerate, sandstone and mudstone from the Uitenhage Group (Thamm and Johnson, 2006), which are overlain with Tertiary terrace gravel and can be found on the upper slopes of the north-western section of the park. The second land type is mainly tertiary and quaternary terrace gravels and covers the majority of the park, central and eastern sections. Within this there are high-level gravel terraces with silcrete and ferricrete outcrops, alluvial flats of loam and sandy loam. The final land type consists of siltstone, shales and sandstones of the Bokkeveld and Witteberg Group, these are found along the Breede River section in the western corner of the park. Soils comprise mainly of alluvial sand along the low-lying areas and undeveloped podzols in the main portion of the park. (Chief Director of Survey and Land Information 1993).
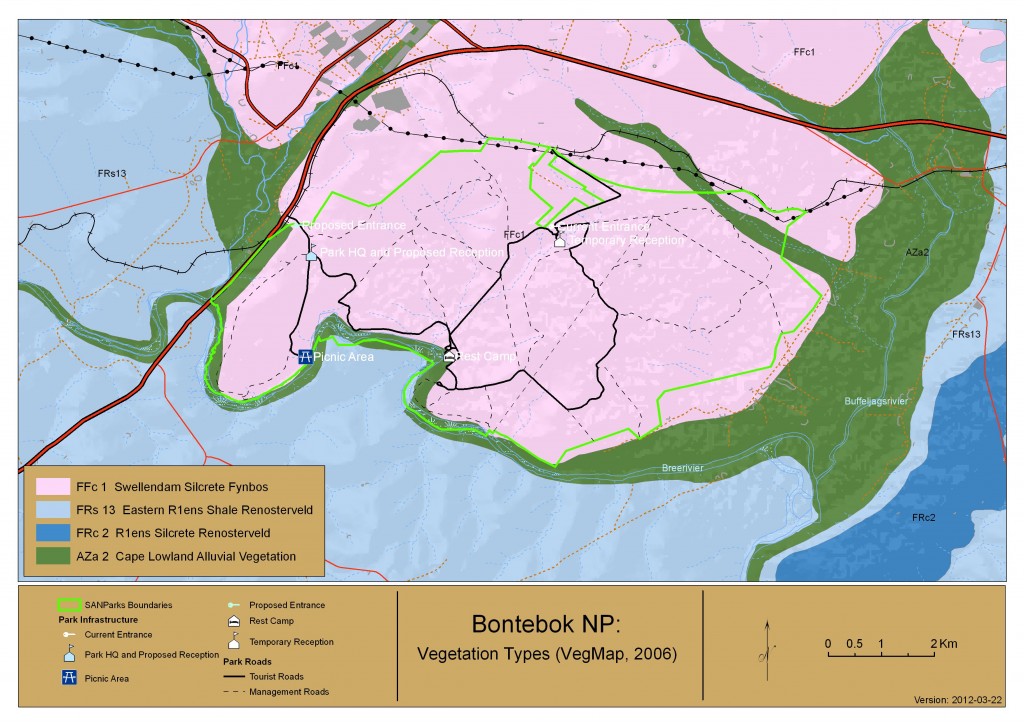
The vegetation and flora of the Park
Originally established to protect the threatened Bontebok antelope the Bontebok National Park is situated in the Cape Flora Region, a Global Biodiversity Hotspot (Goldblatt and Manning, 2000). BNP occurs in an ecotone (transition zone) between Fynbos and Renosterveld and the national vegetation map lists it as Swellendam Silcrete Fynbos (Rebelo et al., 2006) having a mix of both Renosterveld and Fynbos species. The conservation status of this vegetation type is Endangered with the largest portion being formally conserved within BNP. Along the Breede River banks Cape Lowland Alluvial vegetation occurs in the park and is listed as Critically Endangered (Driver et al., 2005), only 1% is formally conserved. A total of 650 floral species are listed as occurring in BNP, from 280 genera and 85 families (Kraaij, 2011). Of these twenty-nine species are listed on the Global threatened species list and a further 23 are recognised as species of conservation concern. The families with the largest species occurrence in the park are the Astercaeae, Iridaceae and Fabaceae (Kraaij, 2011), while the families of Asphodelaceae, Crassulaceae, Poaceae and Cyperaceae were well overrepresented in the park. There are also two species endemic to the park (Aspalathus burchelliana and Diosma fallax) and largest of only 2 populations of Erica filamentosa can be found in Bontebok. Succulents only comprised 8% of the total growth forms within the park with geophtyes being the majority (23%) (Kraaij, 2011). BNP flora is one of the richest in the region and conserves flora that are not conserved elsewhere. It is an area of high conservation priority and diversity and forms a major part in the conservation of Renosterveld in the Overberg region.
The scope and coverage of this report
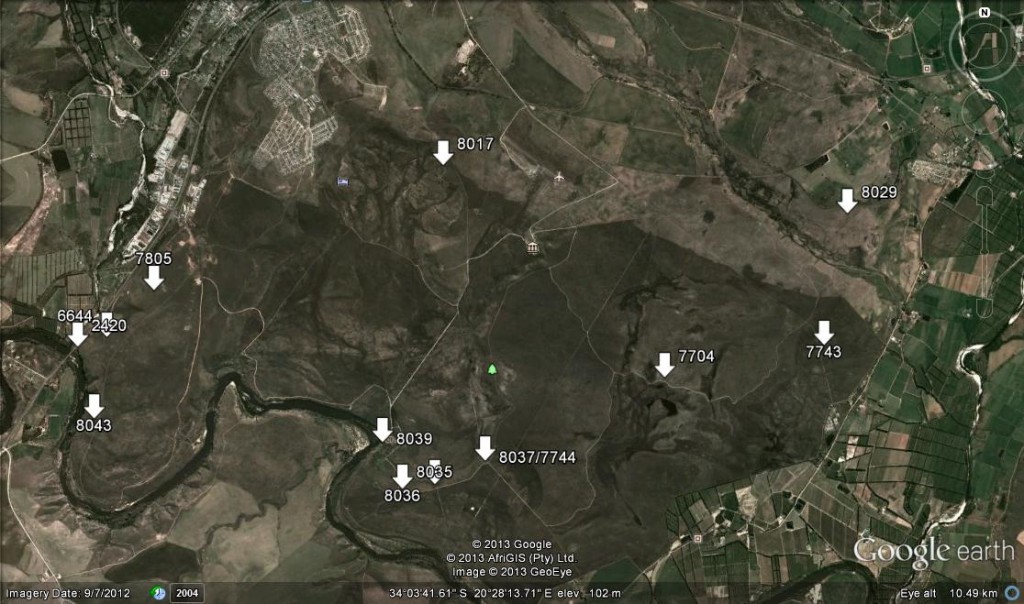
Three of the Haworthia species are known from single populations, and these are indicated on the distribution map (see map 1).
The report therefore covers those species with multiple records, recording their whereabouts and the pictorial plant body and flower variations. Localities are numbered according to M.B. Bayer accession numbers.
A. Haworthia mirabilis.
Accessions 6644, 7704, 7744, 7805, 8035, 8043.
In Jan 2013 population 7704 was in flower, buds were observed at 8035 but not at 7744.
As in my flower report (Update Vol 8) the flowers are not convincingly different from those of H. floribunda. The variation of the plants in especially 7704 and 7805 suggest introgression with H. floribunda.
- MBB6644 – Haworthia mirabilis

















Flower profiles and faces











































2. MBB7704 – Haworthia mirabilis




















































































3. MBB774 – Haworthia mirabilis


4. MBB7805 – Haworthia mirabilis













Flower profiles, faces, and bud







5. MBB8035 – Haworthia mirabilis








6. MBB8043 – Haworthia mirabilis
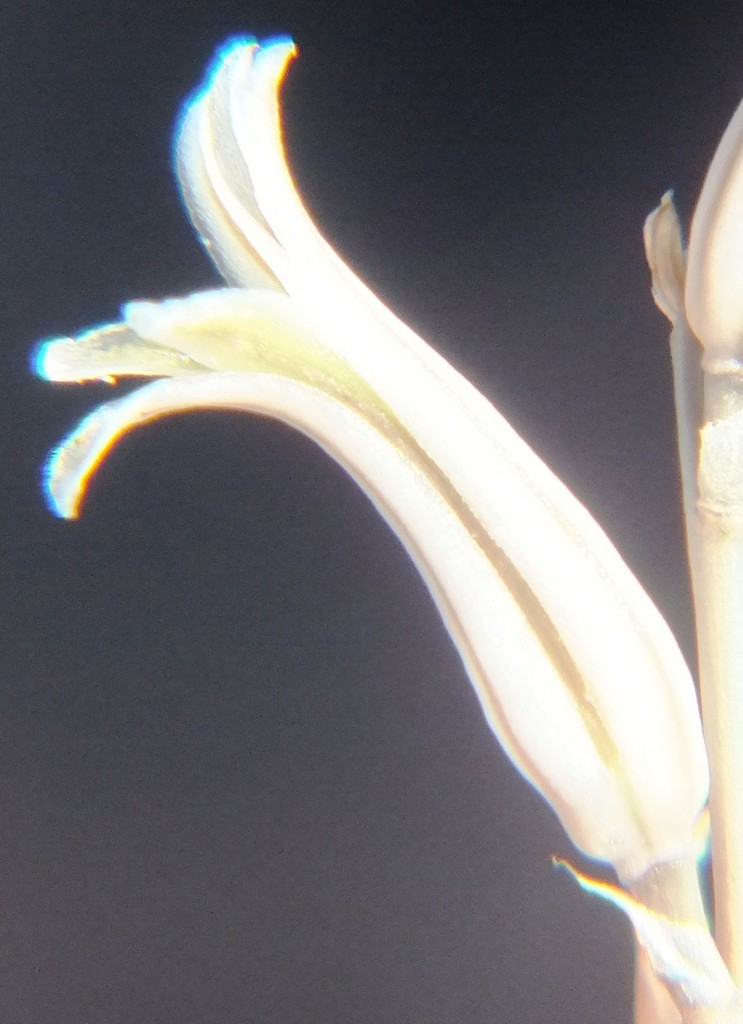

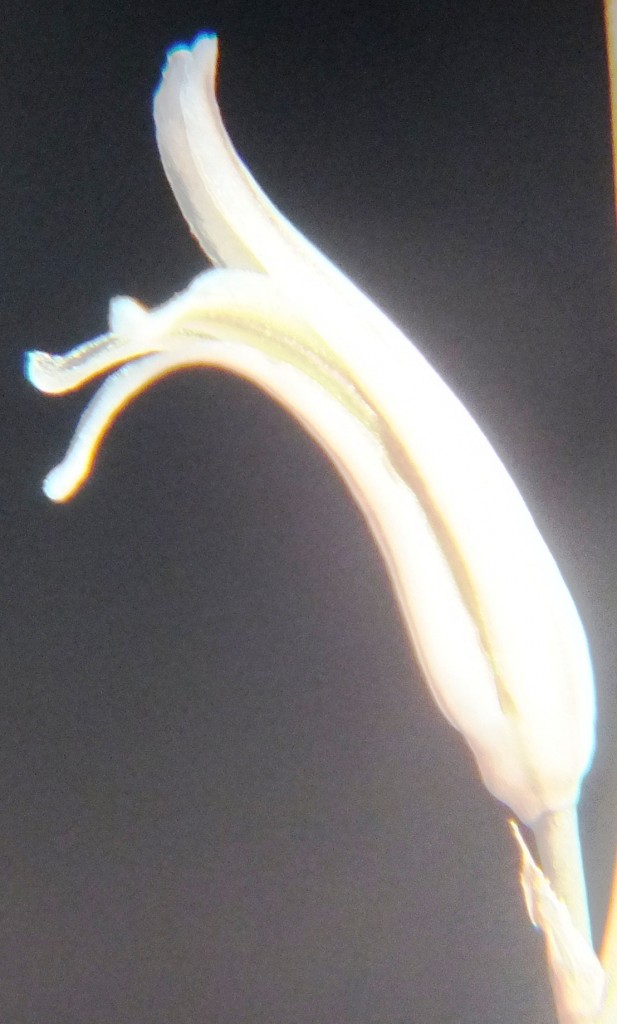
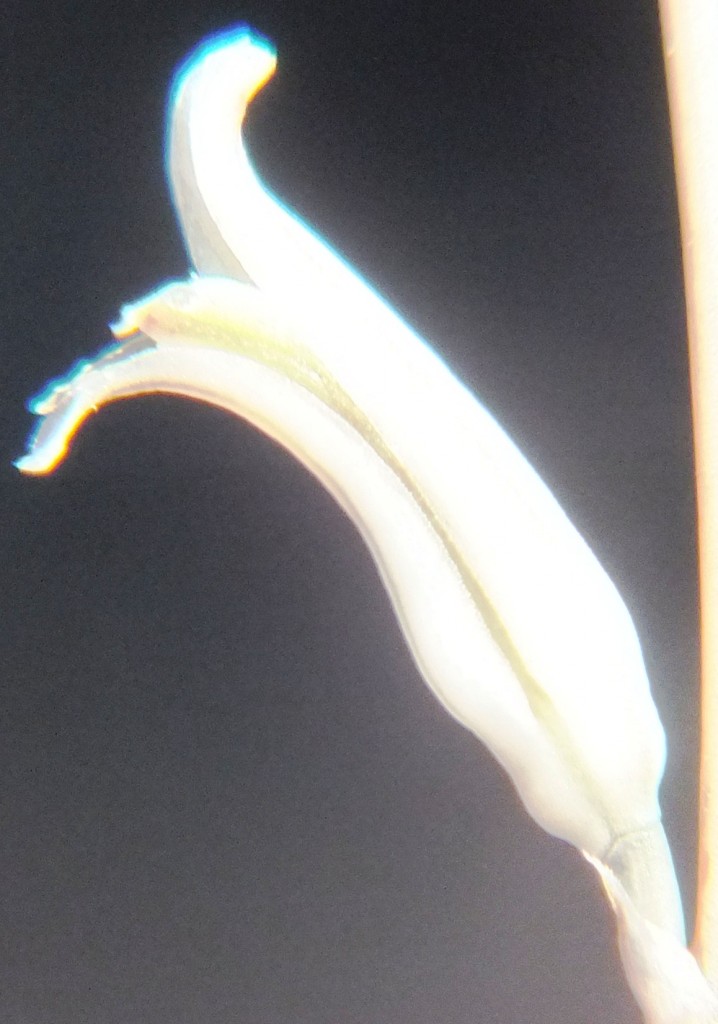

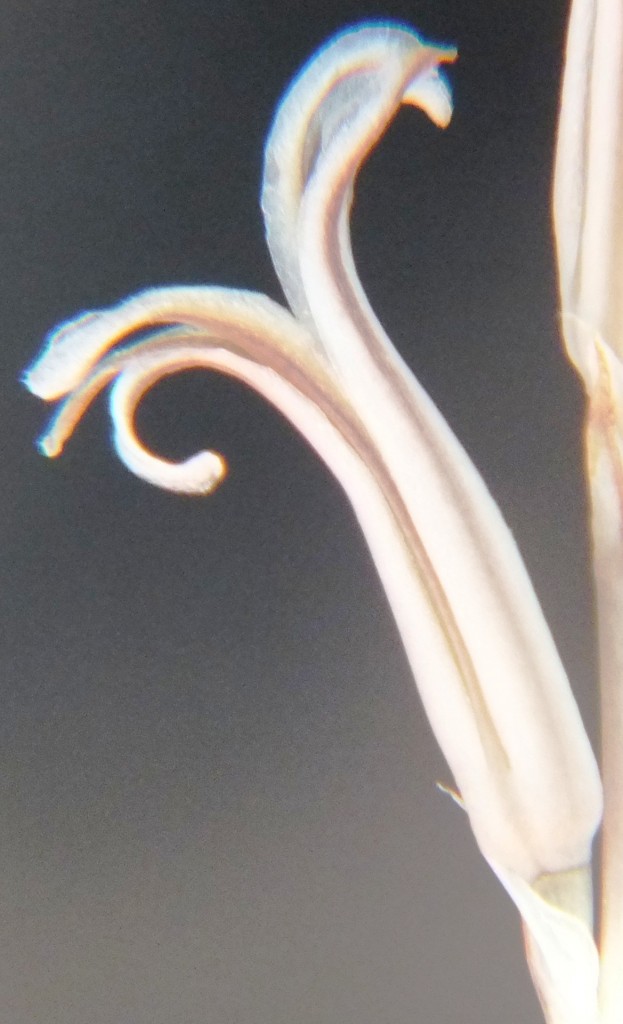
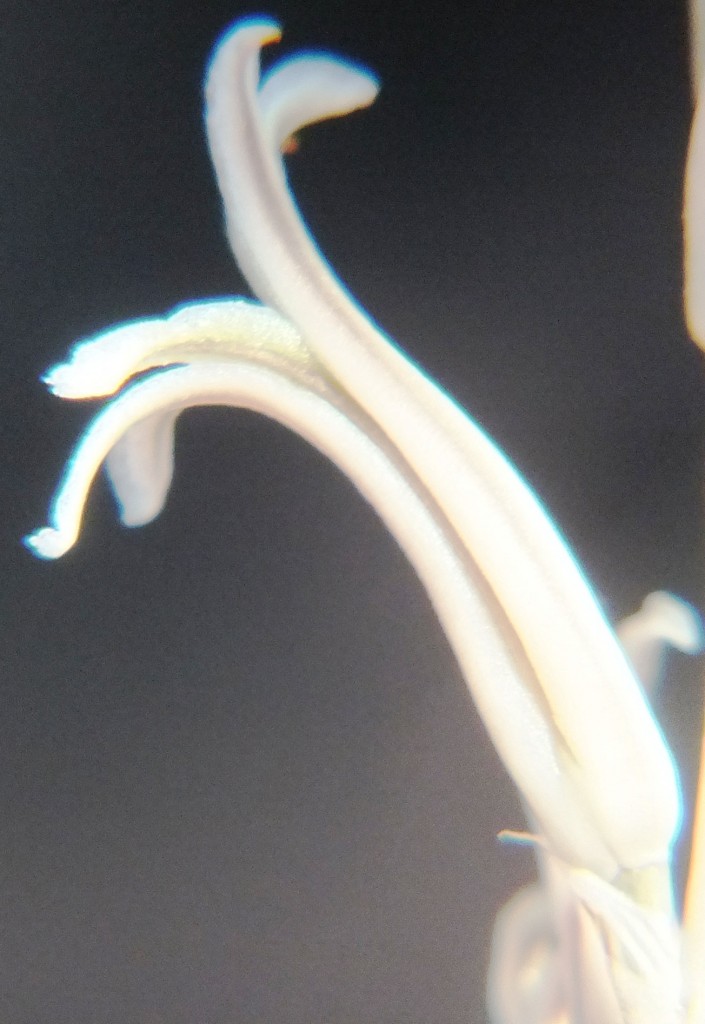
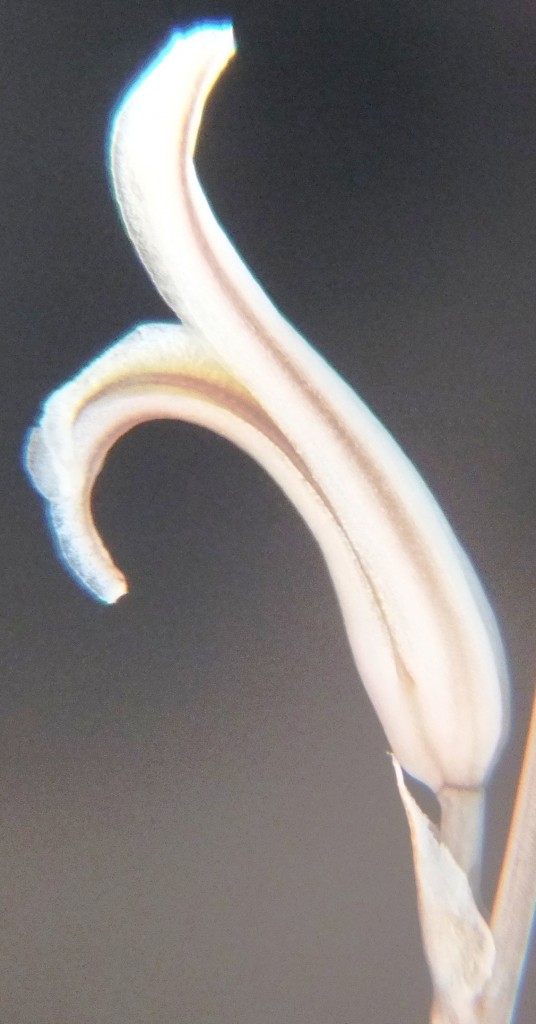




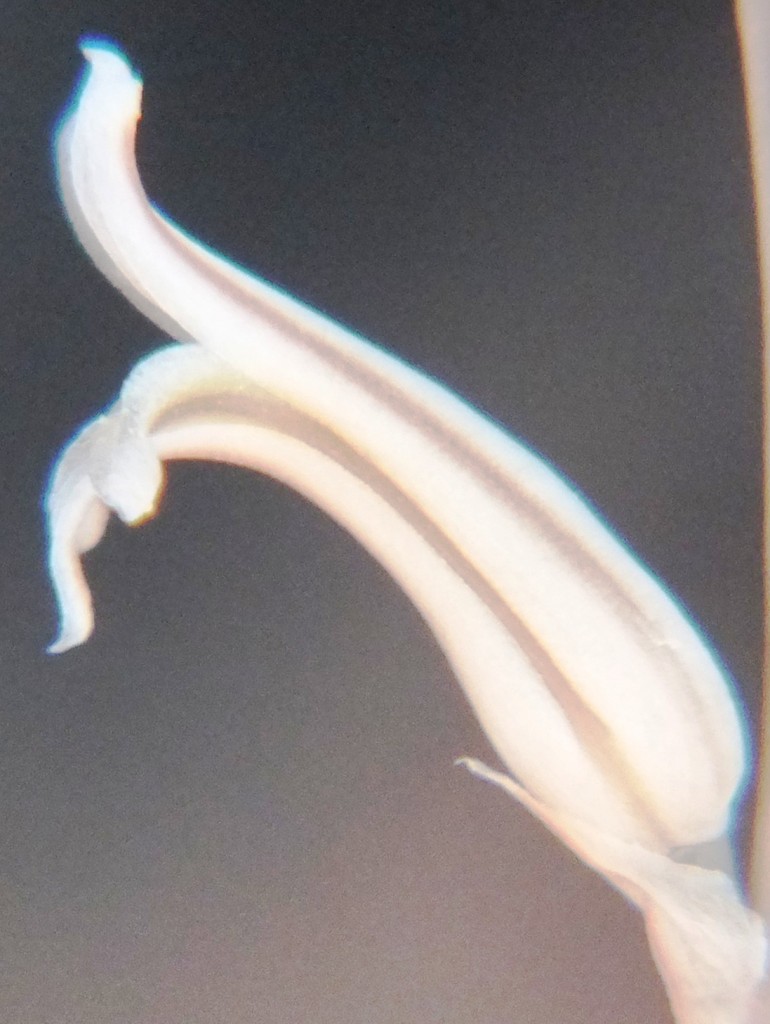

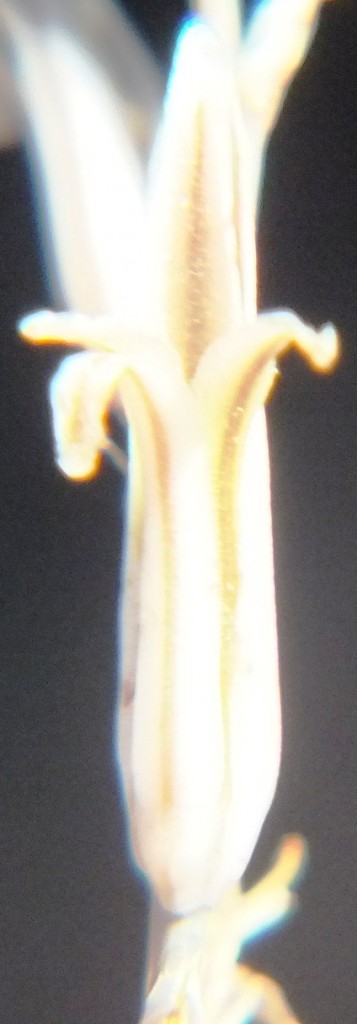


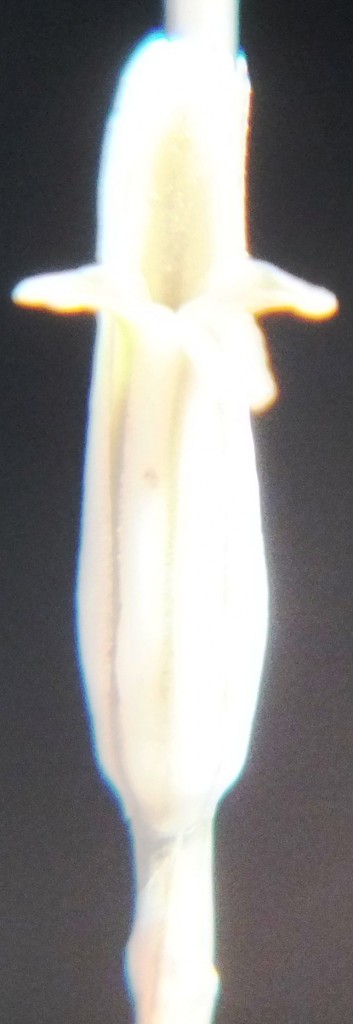
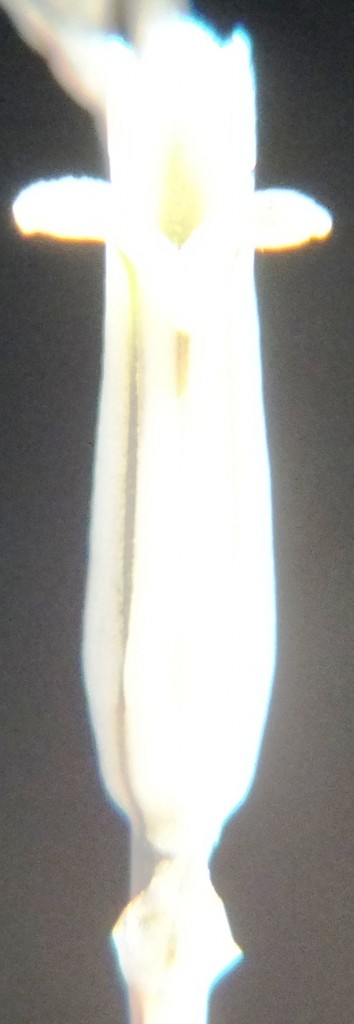








B. Haworthia floribunda.
Accessions 8017. In flower Jan 2013.
A record 3439 exists for the Park, but the precise whereabouts is not recorded. The plant colour and form do not accord with 8017.
7. MBB8017 – Haworthia floribunda




Flower profiles and faces.






































Habitat requirements of Haworthia can be so specific as to defy my describing them. Here is a picture looking north of the habitat for 8017 H. cf floribunda. The rocky ridge extends a long way further north and then west, as well as a long way south. Much of it is of course unexplored in enough detail to know if there are Haworthia there or not. At the southern end of the ridge about 1km away H. mirabilis occurs. To the west the ridge continues, rises and then transforms into steep but low cliffs along the Breede River running northwards. On those cliffs one finds H. retusa (turgida). H. mirabilis is again present on north slopes east of the cliffs. H. venosa is on low river cliffs to the south and it may also be where this ridge abuts the river and turns north.
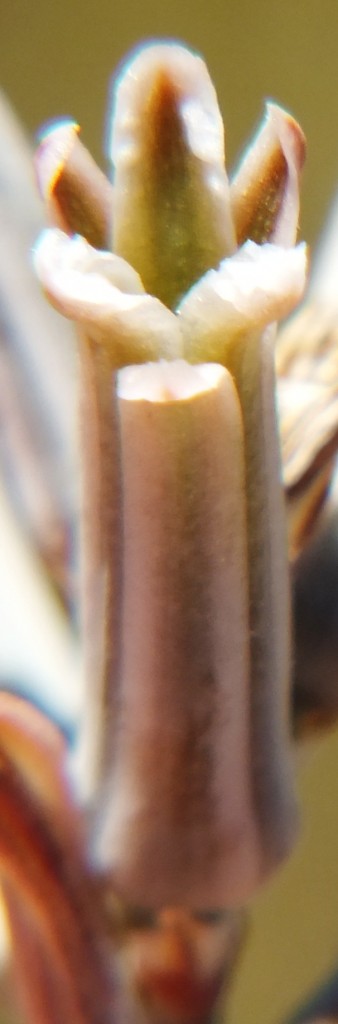
C. Haworthia minima.
Accessions 7743, 8036, 8037
In Jan 2013, a single weak inflorescence was observed at 8036. 8037 was flowering well. No flower or buds were observed at 7743. The plants are not the characteristic blue-green colour of regular H. minima, and the tubercles also seem to be rougher. The flower colour is that of H. minima without the olive-green associated with H. pumila. Note has to be taken of a now non-existent population just south of Swellendam on the N2 that flowered in late Spring with an abnormally large flower, and then a population to the near east at Rotterdam that appears to flower in Summer and where there are hybrids with H. marginata. One cannot exclude the possibility that there is a genetic continuity with either or both, H. marginata and H. pumila.
8. MBB7743 – Haworthia minima









9. MBB8035 – Haworthia minima












10. MBB8037 – Haworthia minima









Flower profiles and faces.






























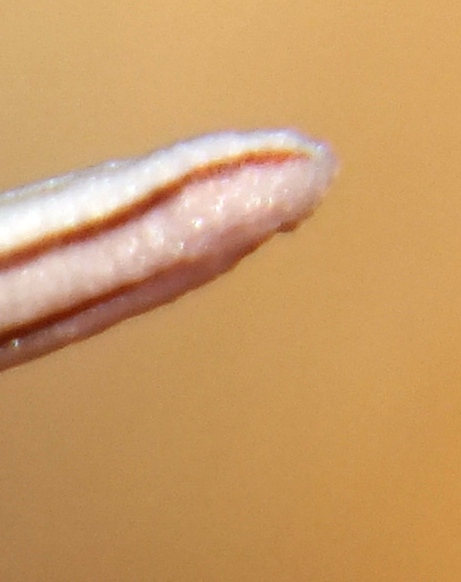
D. Haworthia retusa (turgida).
Accession 2420. This is the cliff-dwelling form of H. retusa and it is only known from the steep riverine cliffs on the northwest boundary of the Park. Expected to be in flower in September.
The significance of the population is the similarity of some clones to H. reticulata that is only known eastwards along the Breede River until Drew from Worcester. It also tends to be a clump-forming species favouring steepish rocky slopes as opposed to its geographic sister species H. herbacea that tends to be more solitary and occupying a wider range of habitat.
11. MBB2420 Haworthia retusa ‘turgida’















References:
DRIVER, A., MAZE, K., ROUGET, M., LOMBARD, A. T., NEL, J., TURPIE, J. K., COWLING, R. M., DESMET, P., GOODMAN, P., HARRIS, J., JONAS, Z., REYERS, B., SINK, K. & STRAUSS, T. (2005) National Spatial Biodiversity Assessment 2004: priorities for biodiversity conservation in South Africa. . Strelitzia 14. South African National Biodiversity Institute.
GOLDBLATT, P. & MANNING, J. (2000) Cape Plants. A conspectus of the Cape Flora of South Africa, Cape Town, National Botanical Institute of South Africa Missouri Botanical Garden Press.
KRAAIJ, T. (2011) The flora of the Bontebok National Park in regional perspective. South African Journal of Botany, 19.
REBELO, A. G., BOUCHER, C., HELME, N., MUCINA, L. & RUTHERFORD, M. C. (2006) Fynbos Biome. IN MUCINA, L. & RUTHERFORD, M. C. (Eds.) The Vegetation of South Africa, Lesotho and Swaziland. Sterlitzia 19. Pretoria, South African National Biodiversity Institute.
THAMM, A. G. & JOHNSON, M. R. (2006) The Cape Supergroup. IN JOHNSON, M. R., ANHAEUSSER, C. R. & THOMAS, J. R. (Eds.) The Geology of South Africa. Johannesburg, The Geological Society of South Africa.



